Our group focuses on the interplay between stress responses, cellular plasticity and cancer. Cellular plasticity is recognized today as a critical feature of cancer cells that enable them to transit between different cellular states and promote tumor growth, disease progression after therapy, and metastasis.
We have previously reported that inducing dedifferentiation with the so-called Yamanaka factors can lead to the development of a variety of tumors. We have also demonstrated that tissue damage –the main driver of cancer– triggers the onset of cellular senescence which then induces dedifferentiation and the acquisition of stem cell properties in vivo.
These findings have important therapeutic implications given that chemotherapy and radiotherapy – cornerstones for the treatment of most cancers – could have the side effect of inducing stemness in non- stem cancer cells and, in turn, possibly contribute to tumor recurrence and cancer cell spread.
Our main objective is to advance insights into the mechanisms and drivers implicated in this process, with the ultimate goal of developing novel therapies based on the inhibition of cancer cell plasticity.
Recent results have demonstrated that some genomic regions, previously considered as non-coding (including lncRNAs), contain small open reading frames encoding for evolutionary conserved, unannotated microproteins. The few that have been identified to date assume key functions in elemental cellular processes, leading to a new level of complexity with major implications – from basic research to the clinical setting.
Over the last few years, our efforts have focused on identifying and characterizing novel cancer microproteins which could be novel actors in carcinogenesis. We have discovered five new cancer microproteins and have obtained compelling evidence in vitro and in vivo that four of them act as novel tumor suppressors, inducing cell cycle arrest, differentiation or inhibition of mesenchymal traits in cancer cells. In addition, using a peptidomics approach, we have identified a set of microproteins secreted by pancreatic tumors, either soluble or secreted in exosomes. These novel microproteins could be crucial cellular messengers for pancreatic cancer metastasis. The identification of tumor-microproteins could be key to advancing insights into cancer physiopathology. Moreover, they could also serve as novel cancer biomarkers for the early detection of disease and patient stratification for tailored therapies, as well as therapeutic targets.
- Advance insights into the interplay between therapy-induced senescence, cellular plasticity and cancer.
- Decipher the molecular mechanisms governing the acquisition of stem cell properties during tumorigenesis and after therapy.
- Discover and characterize novel microproteins involved in cancer cell plasticity.
- Develop novel anti-cancer therapies based on the inhibition of cancer cell plasticity.
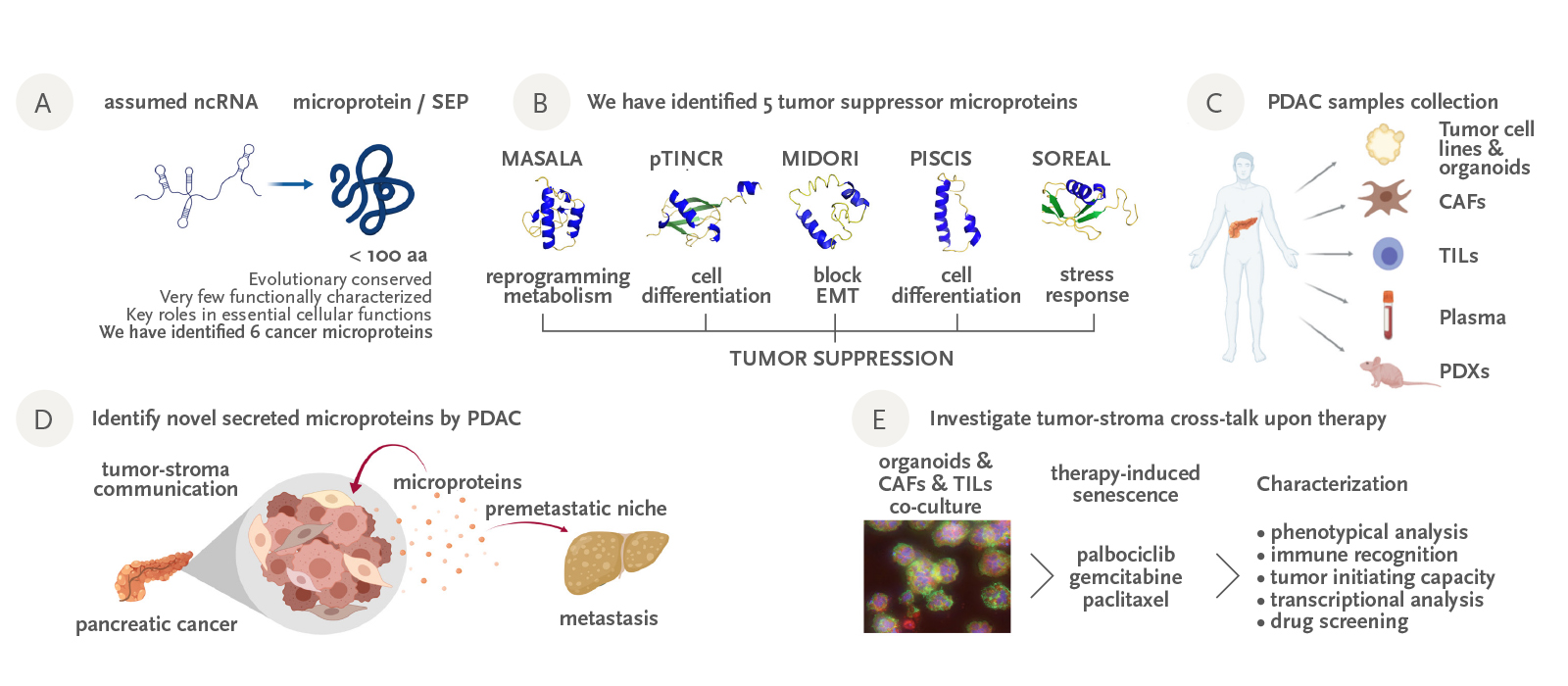
Figure: A) Recent findings have revealed that many genomic regions previously considered as non-coding in fact code for unannotated microproteins; some of them have been shown to be important for cancer. B) Our group has identified 5 novel microproteins with tumor suppressor activities. We have characterized them in vitro and in vivo. C) We have generated a comprehensive patient-match collection of pancreatic cancer samples, that is going to be instrumental for our research. D) We are investigating if cancer cells use unannotated secreted microproteins as intercellular messengers to promote tumor growth and metastasis. E) We are establishing co-cultures of organoids-CAFs-TILs to investigate the impact of therapy in the tumor-stroma cross-talk.
Group Leader
María Abad
Research Assistants
Lluis Palenzuela
Graduate Students
Alba Escriche
Marion Martinez
Most relevant publications 2023
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini CS, Prats N, López-Domínguez JA, Kovatcheva M, Garralda E, Muñoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023 Feb 6;13(2):410-431.
- Pujals M, Mayans C, Bellio C, Méndez O, Greco E, Fasani R, Alemany-Chavarria M, Zamora E, Padilla L, Mitjans F, Nuciforo P, Canals F, Nonell L, Abad M, Saura C, Tabernero J, Villanueva J. RAGE/SNAIL1 signaling drives epithelial-mesenchymal plasticity in metastatic triple-negative breast cancer. Oncogene. 2023 Aug;42(35):2610-2628.
- Álvarez-Sierra D, Sánchez-Gaona N, Cruz Cobo M, Escriche A, Abad M, Gómez-Brey A, Bello I, Caubet E, González Ó, Zafón C, Iglesias C, Moreno P, Petit A, Fernández-Sanmartín MA, Martínez-Gallo M, Pujol-Borrell R. Thyroid cells from normal and autoimmune thyroid glands suppress T lymphocytes proliferation upon contact revealing a new regulatory inhibitory type of interaction independent of PD1/PDL1. J Autoimmun. 2023 Apr;136:103013.
All publications
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini CS, Prats N, López-Domínguez JA, Kovatcheva M, Garralda E, Muñoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023 Feb 6;13(2):410-431.
- Pujals M, Mayans C, Bellio C, Méndez O, Greco E, Fasani R, Alemany-Chavarria M, Zamora E, Padilla L, Mitjans F, Nuciforo P, Canals F, Nonell L, Abad M, Saura C, Tabernero J, Villanueva J. RAGE/SNAIL1 signaling drives epithelial-mesenchymal plasticity in metastatic triple-negative breast cancer. Oncogene. 2023 Aug;42(35):2610-2628.
- Álvarez-Sierra D, Sánchez-Gaona N, Cruz Cobo M, Escriche A, Abad M, Gómez-Brey A, Bello I, Caubet E, González Ó, Zafón C, Iglesias C, Moreno P, Petit A, Fernández-Sanmartín MA, Martínez-Gallo M, Pujol-Borrell R. Thyroid cells from normal and autoimmune thyroid glands suppress T lymphocytes proliferation upon contact revealing a new regulatory inhibitory type of interaction independent of PD1/PDL1. J Autoimmun. 2023 Apr;136:103013.
- Boix O, Martinez M, Vidal S, Giménez-Alejandre M, Palenzuela L, Lorenzo-Sanz L, Quevedo L, Moscoso O, Ruiz-Orera J, Ximénez-Embún P, Ciriaco N, Nuciforo P, Stephan-Otto Attolini C, Albà MM, Muñoz J, Tian TV, Varela I, Vivancos A, Ramón Y Cajal S, Muñoz P, Rivas C, Abad M. pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation. Nat Commun. 2022 Nov 11;13(1):6840.
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Stephan-Otto Attolini C, Prats N, Lopez-Dominguez JA, Kovatcheva M, Garralda E, Munoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M. Cellular senescence is immunogenic and promotes anti-tumor immunity. Cancer Discov. Epub 2022 Oct 27.
- Senís E, Esgleas M, Najas S, Jiménez-Sábado V, Bertani C, Giménez-Alejandre M, Escriche A, Ruiz-Orera J, Hergueta-Redondo M, Jiménez M, Giralt A, Nuciforo P, Albà MM, Peinado H, Del Toro D, Hove-Madsen L, Götz M, Abad M. TUNAR lncRNA Encodes a Microprotein that Regulates Neural Differentiation and Neurite Formation by Modulating Calcium Dynamics. Front Cell Dev Biol. 2021 Dec 31;9:747667.
- Senís E, Mosteiro L, Grimm D, Abad M. A Versatile In Vivo System to Study Myc in Cell Reprogramming. Methods Mol Biol. 2021;2318:267-279.
- Senís E, Mosteiro L, Grimm D, Abad M. Methods Mol Biol. 2021;2318:267-279. doi: 10.1007/978-1-0716-1476-1_14.
- Salazar-Roa M, Trakala M, Alvarez-Fernandez M, Valdes-Mora F, Munoz J, Zapatero-Solana E, Grana O, Peters T,Abad M, Bueno M, Gomez de Cedron M, Fernandez-Piqueras J, De Martino A, Serrano M, Wang D, Clark S, Ortega S and Malumbres M. Transient exposure to miR-203 enhances the differentiation capacity of established pluripotent stem cells. In press. Embo Journal. DOI:10.15252/embj.2019104324
- Merino I, Greco E,Abad M(2020). The microproteome of cancer: from invisibility to relevance.Experimental Cell Research. 2020;392(1):111997. doi:10.1016/j.yexcr.2020.111997
- Senís E, Mosteiro L, Wilkening S, Wiedtke E, Nowrouzi A, Afzal S, Fronza R, Landerer H,Abad M, Niopek D, Schmidt M, Serrano M, Grimm D.AAVvector-mediated in vivo reprogramming into pluripotency.Nat Commun. 2018 Jul 9;9(1):2651. doi: 10.1038/s41467-018-05059-x.
- Abad M, Hashimoto H, Zhou H, Morales MG, Chen B, Bassel-Duby R, Olson EN.Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity. Stem Cell Reports. 2017 Mar 14;8(3):548-560.
- Marión RM, López de Silanes I, Mosteiro L, Gamache B, Abad M, Guerra C, Megías D, Serrano M, Blasco MA.Common Telomere Changes during In Vivo Reprogramming and Early Stages of Tumorigenesis. Stem Cell Reports. 2017 Feb 14;8(2):460-475.
- Gómez-Cabello D, Checa-Rodríguez C, Abad M, Serrano M, Huertas P.CtIP-Specific Roles during Cell Reprogramming Have Long-Term Consequences in the Survival and Fitness of Induced Pluripotent Stem Cells. Stem Cell Reports. 2017 Feb 14;8(2):432-445.
- Mosteiro L, Pantoja C, Alcázar N, Marión RM, Chondronasiou D, Rovira M, Fernández-Marcos PJ, Muñoz M, Blanco-Aparicio C, Pastor J, Gómez-López G, de Martino A, Blasco MA, Abad M and Serrano M. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016 Nov 25;354(6315). pii: aaf4445.
- Adrados I., Larrasa J., Galarreta A., López-Antona I., Menendez C., Abad M., Gil J., Moreno-Bueno G. And Palmero I. The homeoprotein SIX1 controls cellular senescence through the regulation of p16INK4A and differentiation-related genes. Oncogene. 2016 Jul 7;35(27):3485-94
- Vilas JM, Ferreirós A, Carneiro C, Morey L, Da Silva-Álvarez S, Fernandes T, Abad M, Di Croce L, García-Caballero T, Serrano M, Rivas C, Vidal A, Collado M. Transcriptional regulation of Sox2 by the retinoblastoma family of pocket proteins. Oncotarget. 2015 Feb 20;6(5):2992-3002.
- Palla A.R., Piazzolla D., Abad M., Li H., Dominguez O., Schönthaler H.B., Wagner E.F. and Serrano M.Reprogramming activity of NANOGP8, a NANOG family member widely expressed in cancer. Oncogene.2014 May 8;33(19):2513-9
- Abad M, Mosteiro L, Pantoja C, Cañamero M, Rayon T, Ors I, Graña O, Megías D, Domínguez O, Martínez D, Manzanares M, Ortega S, Serrano M.Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013 Oct 17;502(7471):340-5.
- Abad M, Moreno A, Palacios A, Narita M, Blanco F, Moreno-Bueno G, Narita M and Palmero I.The tumor suppressor ING1 contributes to epigenetic control of cellular senescence. Aging Cell. 2011 Feb; 10:158-71.
- Menéndez C, Abad M, Gómez-Cabello D, Moreno A and Palmero I. ING proteins in cellular senescence. Curr Drug Targets. 2009 May;10:406-1.
- Abad M, Menéndez C, Fuchtabuer A, Serrano M, Fuchtbauer E-M and Palmero I.Ing1 mediates p53 accumulation and chromatin modification in response to oncogenic stress.J Biol Chem. 2007 Oct 19;282:31060-7
- Goeman F.,Thormeyer D., Abad M., Serrano M., Schmidt O., Palmero I. and Baniahmad A. Growth inibition by the tumor suppressor p33ING1 in immortalized and primary cells: Involvement of two silencing domains and effect of Ras.Mol Cell Biol. 2005 Jan;25:422-31.
- Tumoral senescence induced by anti-cancer therapies constitutes a novel prognostic biomarker and a therapeutic target.
Reference: PRYCO211023SERR.
Spanish Association Against Cancer – AECC.
Award Period: 9/2021-8/2026
Co-PI: María Abad. - Defining the Role of Exosome-Secreted Micropeptides in Pancreatic Cancer. number: HR18-00256.
Health Research Grant-La Caixa Foundation.
Award Period: 9/2019-8/2021.
PI: Maria Abad. - Identificación y Análisis del Microproteoma del Cáncer de Páncreas: Los Micropéptidos como Nuevas Dianas Terapéuticas y Biomarcadores Tumorales Fundación. XVI Convocatoria de Ayudas a la Investigación en Salud.
Fundación Mutua Madrileña.
01/09/2019-30/08/2022.
PI: Maria Abad. - Mining the Microproteome for New Molecular Targets in Cancer.
number: RTI2018-102046-B-I00.Spanish Ministry of Economy and Competitiveness.
1/2019-12/2021.
PI: Maria Abad.















