
- Published in ESMO Open, results of a phase I first-in-human study co-authored by investigators at the Vall d’Hebron Institute of Oncology (VHIO), show that novel monoclonal antibody MSC-1 is safe and well tolerated in patients with advanced solid tumors.
- This open-label phase I study enrolled 41 patients treated at the Vall d’Hebron University Hospital, Memorial Sloan Kettering Cancer Center, New York (USA), and the Princess Margaret Cancer Center, Toronto (Canada). Based on promising results of this clinical trial, a phase II study of first line MSC-1 in combination with immunotherapy is now underway in patients with metastatic pancreatic cancer.
- In parallel, findings of a preclinical study co-directed by VHIO’s Joan Seoane published ahead of print in Clinical Cancer Research point to the promise of LIF as a novel drug target in immune-oncology. Data show that combined MSC-1 with anti-PD1 achieves a strong anti-tumor response and durable disease-free survival in mouse models of various tumor types.
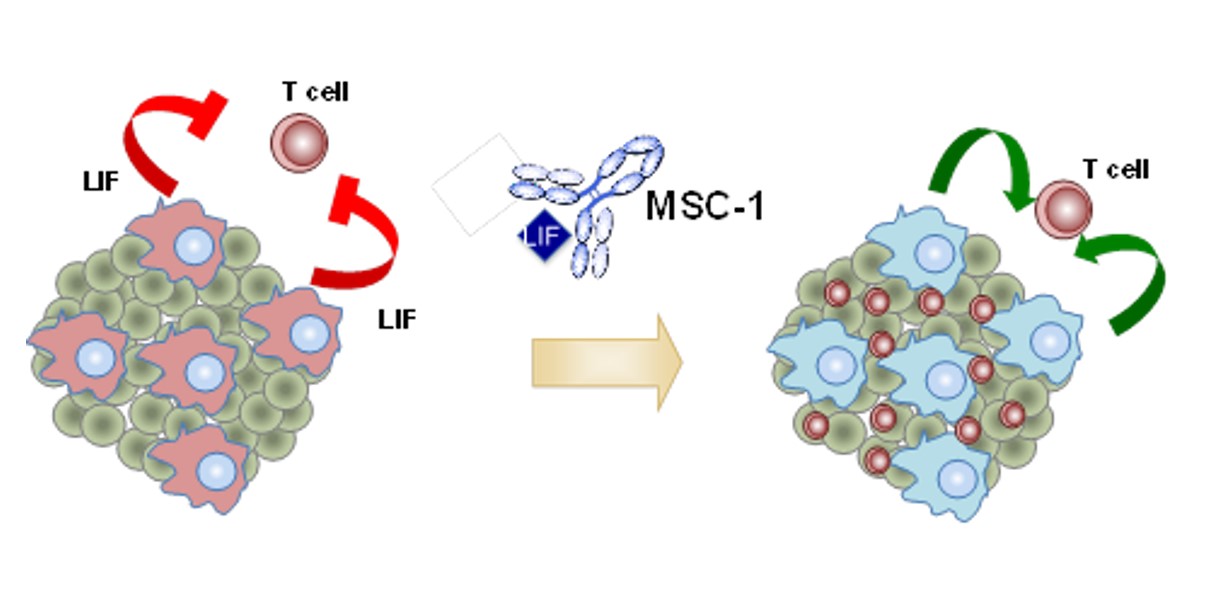
- The clinical study, through the analysis of pre- and on treatment tumor biopsies, and the preclinical study evidence how MSC-1 modulates the tumor microenvironment and converts immunosuppressive tumor-associated macrophages (TAMs) into immunostimulatory macrophages and induces immune cell (T-cell) infiltration in tumors.
Previous research led by Joan Seoane, co-Director of Preclinical and Translational Research at the Vall d’Hebron Institute of Oncology (VHIO) established the role leukemia inhibitor factor (LIF) in oncogenesis as a promoter of cancer progression by regulating the tumor microenvironment and inducing self-renewal in tumor-initiating cells (1).
Joan Seoane and his group at VHIO were the first to preclinically establish a link between this multi-functional protein and cancer and showed that LIF blockade eliminates cancer stem cells and prevents disease progression and recurrence, as well as described its implication in the immune system’s anti-cancer response.
After several years’ research and validating LIF’s promise as a therapeutic target in preclinical and experimental models, the first-in-class MSC-1 therapeutic LIF neutralizing antibody was developed by Joan Seoane’s team and VHIO-born spin-off Mosaic Biomedicals that he co-founded back in 2012.
“MSC-1’s transition to the clinic and translation into benefits for cancer patients promises an important addition to the current arsenal of powerful anti-cancer weaponry. With a dual mechanism of action to eliminate cancer stem cells and activate the immune system, this LIF inhibitor has been clinically evaluated as monotherapy and is now being assessed in combination with immunotherapy,” said Joan Seoane, an ICREA Research Professor and Professor at the Universitat Autònoma de Barcelona (UAB).
Recently published in ESMO Open (2), results of a phase I first-in-human study led by colleagues at the HonorHealth Research and Innovation Institute, Scottsdale, and the Memorial Sloan Kettering Cancer Center – MSKCC, New York (USA), and co-authored by VHIO investigators including Joan Seoane and VHIO’s Director Josep Tabernero, show that this novel monoclonal antibody MSC-1 is safe and well tolerated as monotherapy in patients with advanced, unresectable solid tumors.
This open-label phase I clinical trial enrolled 41 patients with different tumor types including pancreatic, colorectal, head and neck, ovarian, and prostate cancer, who were treated at the Vall d’Hebron University Hospital (Vall d’Hebron Barcelona Hospital Campus), Memorial Sloan Kettering Cancer Center, New York (USA), and the Princess Margaret Cancer Center, Toronto (Canada). These patients were heavily pre-treated with progressive disease after standard-of-care. The primary objectives of this study were to evaluate the safety and tolerability of MSC-1, determine the recommended phase II study dose, and establish the pharmacodynamics and pharmacokinetics of the MSC-1 novel monoclonal antibody.
“MSC-1 was very well tolerated across doses, with no serious treatment related adverse events reported. In addition, we performed tumor biomarker analysis in patient biopsies to advance insights into the mechanism of action of this drug,” observed Joan Seoane, a co-author of this study.
LIF as a novel drug target in cancer therapy
The LIF recombinant protein assumes an important role in a wide array of physiological and pathological processes. Previous research led by Joan Seoane (1) exposed the parallels between embryogenesis and cancer. During embryogenesis LIF protects the embryo from the mother’s immune system. Cancer seizes on this molecular mechanism induced by LIF and uses it for its own gain.
LIF is aberrantly expressed in some cancers and subsequently shields tumors from the patient’s own immune system; in the same way that it protects the embryo. When expressed in cancer it promotes cancer stem cell proliferation and plays a key role in tumor growth and disease progression. The MSC-1 antibody (now AZD0171), was designed and developed to block LIF signaling to unleash the antitumor immune response, eliminate cancer stem cells and prevent disease progression and recurrence in patients with advanced solid tumors.
“Data from this first-in-human phase I clinical trial also show preliminary evidence of immunoactivating activity. Biomarkers of immune activation identified in the tumor microenvironment of patients’ biopsies support the therapeutic hypothesis of MSC-1,” said Seoane.
Results of this present study point to LIF as a promising drug target for advanced solid tumors, and a phase II clinical trial of MSC-1 in combination with anti-PD-L1 is currently underway in patients with advanced pancreatic cancer, the results of which are expected to establish the antitumor efficacy of this novel compound.
Notably, while the main objective of this clinical trial was not to evaluate the efficacy of MSC-1, the investigators observed that this agent halted tumor growth in 9 patients (23.7%). In one patient with advanced pancreatic cancer who had received four prior lines of therapy, a 40% tumor reduction was achieved in one of the lesions.
LIF and the modulation of immune system response
In parallel to the above-mentioned study, results of research co-led by Joan Seoane and investigators of his Gene Expression and Cancer Group have now published ahead of print in Clinical Cancer Research (3), a journal of the American Association of Cancer Research (AACR). The reported data further support the MSC-1 as a therapeutic compound for the treatment of cancer and point to LIF as a novel therapeutic target for cancer immunotherapy.
Tumors develop in dynamic and complex microenvironments. This surrounding terrain, that influences their growth, invasion, and metastasis, is populated by a variety of cell types including macrophages. Under normal conditions, these specialized cells play a key role in the detection, phagocytosis and destruction of bacteria and other harmful organisms.
“In the tumor microenvironment, tumor-associated macrophages can be immunosuppressive with the ability of switching off the immune response against cancer cells and thus promote disease progression. Alternatively, they can act as immunostimulators that induce activation of the immune response, putting the brakes on tumor growth,” explained Joan Seoane, a co-corresponding and senior author of this present study.
 The investigators evaluated LIF expression levels across 7,769 patients representing 22 solid tumor indications profiled as part of U.S. National Cancer Institute (NCI) – National Human Genome Research Institute’s (NHGRI) Cancer Genome Atlas (TCGA) cancer genomics program and studied the type of identified tumor-associated macrophages (TAMs); immunosuppressive or immunostimulatory.
The investigators evaluated LIF expression levels across 7,769 patients representing 22 solid tumor indications profiled as part of U.S. National Cancer Institute (NCI) – National Human Genome Research Institute’s (NHGRI) Cancer Genome Atlas (TCGA) cancer genomics program and studied the type of identified tumor-associated macrophages (TAMs); immunosuppressive or immunostimulatory.
“We also explored the relationship between LIF expression and patient outcomes in the TCGA cohort and a recently described cohort of patients treated with immune checkpoint inhibitors. Our results show that high and low levels of LIF expression significantly associated with worse and better overall survival, respectively, and expose a link between LIF expression and resistance to immunotherapy,” added Ester Bonfill-Teixidor and Raffaella Iurlaro, Post-Doctoral Investigators of Joan Seoane’s Group, and co-authors of this study.
Data point to high levels of LIF expression as a significant predictor of a poor prognosis and indicate that LIF contributes to the immunosuppressive nature of macrophages as a driver of tumor growth through the deactivation of the immune response to cancer. The authors have also preclinically evidenced that MSC-1 inhibition of LIF transforms macrophages from a pro-tumorigenic immunosuppressive state to an antitumor immunostimulatory state and promotes T cell infiltration in tumors, triggering an anti-cancer immune response.
“We have observed that, at least in part, the change in macrophage characteristics and increased T-cell infiltration to promote anti-tumor immunity potentiates MSC-1 as a therapeutic compound for the treatment of cancer,” concluded Joan Seoane.
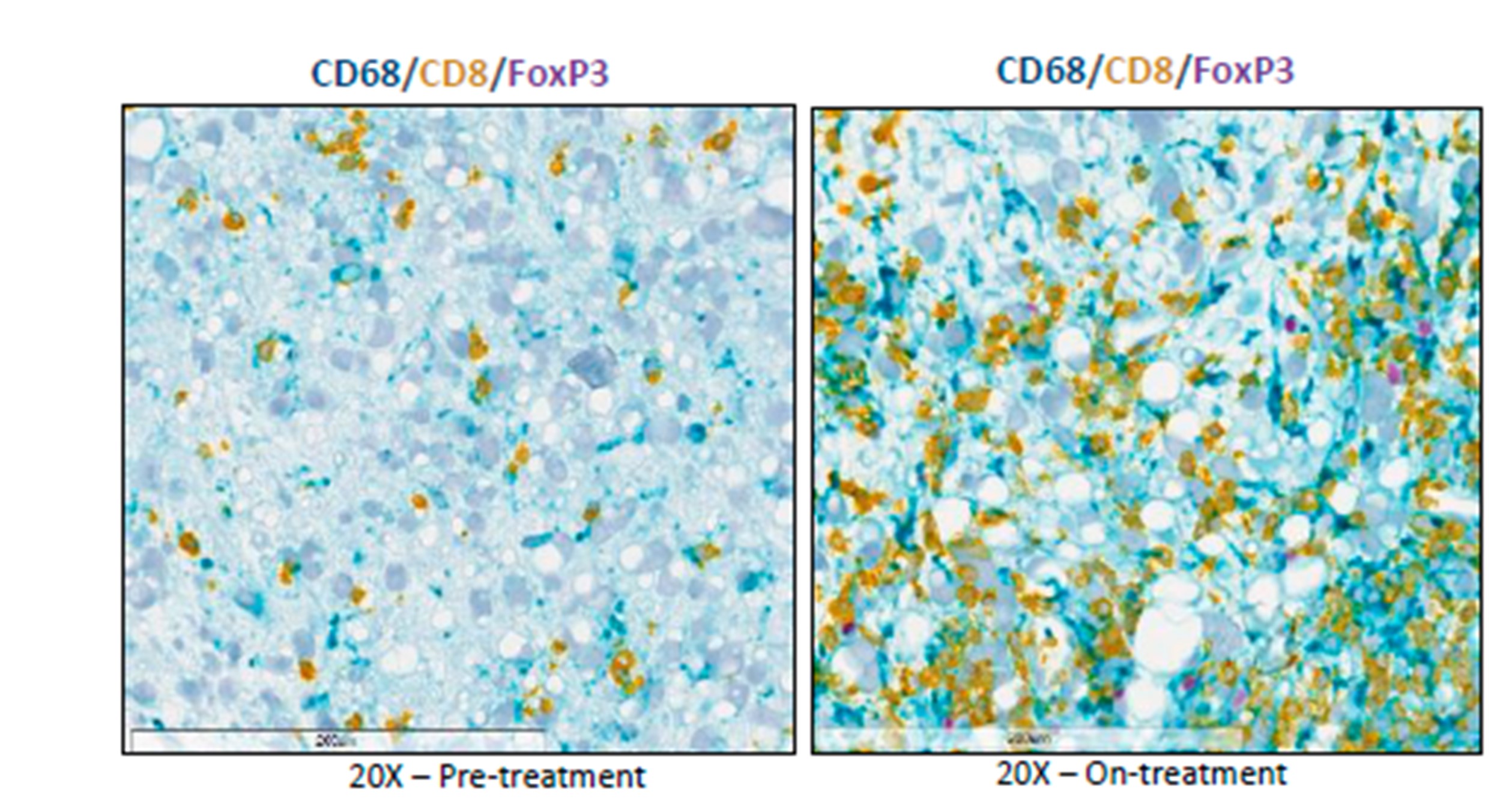
Their preclinical data were validated in the above-mentioned phase I, first-in-human study of MSC-1 through the characterization of biopsies pre- and post-treatment. Using these patient samples, the investigators observed how MSC-1 modulates the tumor microenvironment and converts immunosuppressive tumor-associated macrophages (TAMs) into immunostimulatory macrophages and induces immune T-cell infiltration in tumors.
—-
Joan Seoane and his team’s preclinical work and their development of clinical trials to evaluate MSC-1 in cancer patients was supported, in part, by funding received from the Fundación Asociación Española contra el Cáncer (AECC), European Research Council (ERC), Fundación Fero, Fundació Privada CELLEX, Fundación BBVA, and the “La Caixa” Foundation.
References:
- Pascual-García M, Bonfill-Teixidor E, Planas-Rigol E, Rubio-Perez C, Iurlaro R, Arias A, Cuartas I, Sala-Hojman A, Escudero L, Martínez-Ricarte F, Huber-Ruano I, Nuciforo P, Pedrosa L, Marques C, Braña I, Garralda E, Vieito M, Squatrito M, Pineda E, Graus F, Espejo C, Sahuquillo J, Tabernero J, Seoane J. LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+T cell tumor-infiltration impairing anti-PD1 therapy. Nat Commun. 2019 Jun 11;10(1):2416.
- Borazanci E, Schram AM, Garralda E, Brana I, Vieito Villar M, Spreafico A, Oliva M, Lakhani NJ, Hoffman K, Hallett RM, Maetzel D, Hua F, Hilbert J, Giblin P, Anido J, Kelly A, Vickers PJ, Wasserman R, Seoane J, Siu LL, Hyman DM, Hoff DV, Tabernero J. Phase I, first-in-human study of MSC-1 (AZD0171), a humanized anti-leukemia inhibitory factor monoclonal antibody, for advanced solid tumors. ESMO Open. 2022 Aug;7(4):100530. doi: 10.1016/j.esmoop.2022.100530. Epub 2022 Jul 31. PMID: 35921760; PMCID: PMC9434412.
- Hallett R, Bonfill-Teixidor E, Iurlaro R, Arias A, Raman S, Bayliss PE, Egorova O, Neva-Alejo A, McGray AR, Lau E, Bosch A, Beilschmidt M, Maetzel D, Fransson J, Huber-Ruano I, Anido J, Julien JP, Giblin PA, Seoane J. Therapeutic targeting of LIF overcomes macrophage mediated immunosuppression of the local tumor microenvironment. Clin Cancer Res. 2022 Nov 28:CCR-21-1888. doi: 10.1158/1078-0432.CCR-21-1888. Epub ahead of print. PMID: 36441800.


















