
Immunotherapies against cancer exploit the immune system to more effectively attack disease. Clinical studies have shown that immune checkpoint inhibitors and T-cell-based therapies can mediate tumor regression in cancer patients with metastatic disease. Thus, in addition to surgery, radiation therapy and chemotherapy, immunotherapy is increasingly representing the fourth pillar of anti-cancer therapy across various tumor types.
Immunotherapies exploit the immune system to attack cancer. Clinical studies have shown that immune checkpoint inhibitors and T-cell-based therapies can mediate tumor regression in patients with metastatic disease. Thus, in addition to surgery, radiation therapy and chemotherapy, immunotherapy has become the fourth pillar of anti-cancer therapy. Our group focuses on better understanding the naturally occurring T-cell response to cancer and establishing ways to exploit these antitumor responses to develop more effective, powerful, and personalized immunotherapies against cancer.
Accumulating evidence supports that neoantigens play an important role in the clinical efficacy of cancer immunotherapies. Thanks to the support received from the Fundación BBVA’s Comprehensive Program of Cancer Immunotherapy & Immunology – CAIMI, as well as other funding agencies, and through our group’s long-standing collaboration with Elena Garralda, Principal Investigator of VHIO’s Early Clinical Drug Development Group, and Director of our Research Unit for Molecular Therapy of Cancer (UTIM) – CaixaResearch, we received authorization from the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS – Spanish Regulatory Agency) in May 2021 to initiate a phase I clinical trial to test the safety and tolerability of neoantigen-selected TILs.
In this ongoing clinical trial we are using a highly personalized approach (see Figure) to screen for T-cell mediated recognition of mutated antigens using autologous antigen presenting cells that can process and present in all the potential human leukocyte antigen (HLA). In this pilot clinical study funded by the Instituto de Salud Carlos III – ISCIII (Carlos III Health Institute), we aim to treat up to 10 patients with epithelial cancers and melanoma refractory to standard therapies. By enriching for neoantigen-reactive lymphocytes, we hope to extend the efficacy of TIL therapy beyond melanoma.
Our group’s work has demonstrated that tumor-reactive T cells can frequently be detected circulating in the blood of cancer patients, regardless of the specific tumor type. The ability to track and monitor tumor-reactive CD8+ and CD4+ T cells in blood has tremendous therapeutic potential, but it is more challenging due to their lower prevalence. Our group has contributed to defining a phenotypic signature that can guide the identification and enrichment of tumor- and neoantigen-reactive T cells from the blood of cancer patients and we aim to leverage this to develop minimally-invasive T-cell therapies to treat cancer patients. In addition, we now aim to conduct extensive profiling of the peripheral blood T-cell response at the single cell level in patients treated with immune checkpoint inhibitors to better understand the determinants of response.
In addition to the above-mentioned, our group is also interested in developing high-throughput technologies to better understand the landscape of tumor antigens that render tumors susceptible to immune attack, shared neoantigens as well as mechanisms of resistance to T-cell cytotoxicity.
- Develop minimally-invasive personalized T-cell therapies derived from peripheral blood.
- Track tumor-antigen specific T cells immune-dynamics in patients treated with immunotherapy to understand determinants of response and toxicity.
- Investigate novel strategies to rapidly identify shared neoantigens that can serve as targets for TCR gene-engineered therapies.
- Mine tumor cell intrinsic mechanisms of resistance to T-cell mediated cytotoxicity.
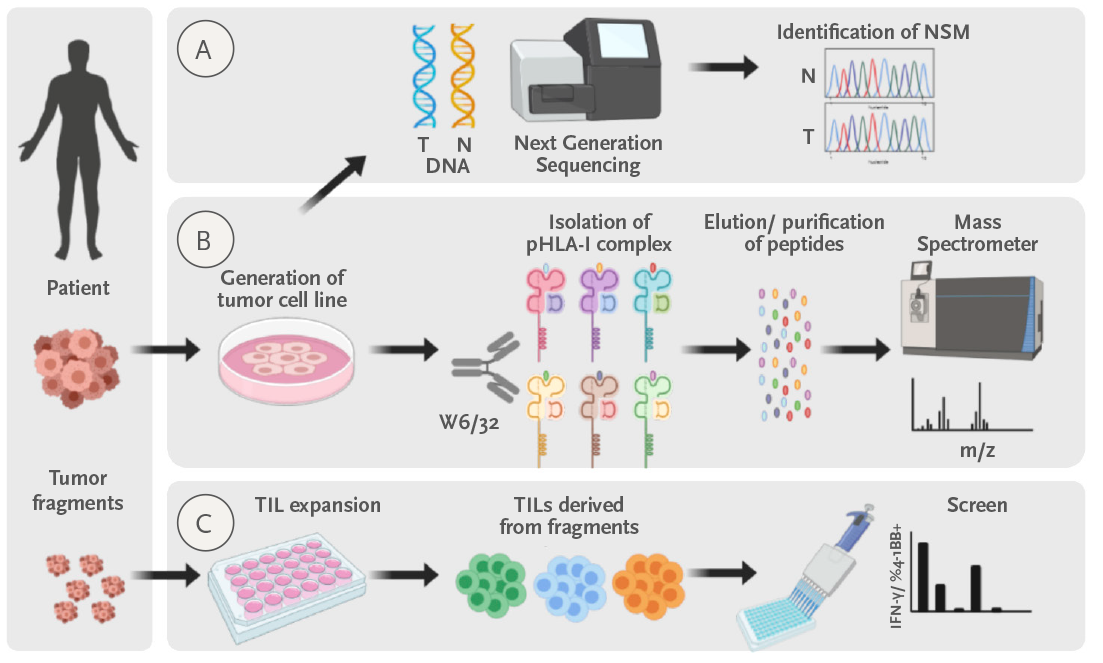
Figure: Personalized approach to identify tumor and neoantigen- specific TILs. a) We sequence normal and tumor DNA to identify all the non-synonymous mutations. b) In parallel we attempt to generate a tumor cell line. When generated, we isolate the peptide-MHCI complexes and we identify the peptides presented by MHCI by the tumor cell line by Mass spectrometry. c) Finally, we screen the TILs expanded from the tumor for recognition of the candidate neoantigen peptides identified in a) or elluted from MHCI in b).
Group Leader
Alena Gros
Post-Doctoral Fellows
Pierre Levy
Andrea Garcia-Garijo
Endika Prieto
Graduate Students
Judit Díaz
Immaculada Creus
Anna Yuste
Johanna Kusnick
Technicians
Marina Arroyo
Carla Brujas
Albert Marin
Lab Manager
Noelia Alcazar
- Lozano-Rabella M, Garcia-Garijo A, Palomero J, Yuste-Estevanez A, Erhard F, Farriol-Duran R, Martín-Liberal J, Ochoa-de-Olza M, Matos I, Gartner JJ, Ghosh M, Canals F, Vidal A, Piulats JM, Matías-Guiu X, Brana I, Muñoz-Couselo E, Garralda E, Schlosser A, Gros A. Exploring the Immunogenicity of Noncanonical HLA-I Tumor Ligands Identified through Proteogenomics. Clin Cancer Res. 2023 Jun 13;29(12):2250-2265.
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini CS, Prats N, López-Domínguez JA, Kovatcheva M, Garralda E, Muñoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M. Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity. Cancer Discov. 2023 Feb 6;13(2):410-431.
- Puig-Saus C, Sennino B, Peng S, Wang CL, Pan Z, Yuen B, Purandare B, An D, Quach BB, Nguyen D, Xia H, Jilani S, Shao K, McHugh C, Greer J, Peabody P, Nayak S, Hoover J, Said S, Jacoby K, Dalmas O, Foy SP, Conroy A, Yi MC, Shieh C, Lu W, Heeringa K, Ma Y, Chizari S, Pilling MJ, Ting M, Tunuguntla R, Sandoval S, Moot R, Hunter T, Zhao S, Saco JD, Perez-Garcilazo I, Medina E, Vega-Crespo A, Baselga-Carretero I, Abril-Rodriguez G, Cherry G, Wong DJ, Hundal J, Chmielowski B, Speiser DE, Bethune MT, Bao XR, Gros A, Griffith OL, Griffith M, Heath JR, Franzusoff A, Mandl SJ, Ribas A. Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature. 2023 Mar;615(7953):697-704.
- García-Mulero S, Fornelino R, Punta M, Lise S, Varela M, Del Carpio LP, Moreno R, Costa-García M, Rieder D, Trajanoski Z, Gros A, Alemany R, Piulats JM, Sanz-Pamplona R. Driver mutations in GNAQ and GNA11 genes as potential targets for precision immunotherapy in uveal melanoma patients. Oncoimmunology. 2023 Oct 24;12(1):2261278.
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Stephan-Otto Attolini C, Prats N, Lopez-Dominguez JA, Kovatcheva M, Garralda E, Munoz J, Caron E, Abad M, Gros A, Pietrocola F, Serrano M. Cellular senescence is immunogenic and promotes anti-tumor immunity. Cancer Discov. Epub 2022 Oct 27.
- Palomero J, Panisello C, Lozano-Rabella M, Tirtakasuma R, Díaz-Gómez J, Grases D, Pasamar H, Arregui L, Dorca Duch E, Guerra Fernández E, Vivancos A, de Andrea CE, Melero I, Ponce J, Vidal A, Piulats JM, Matias-Guiu X, Gros A. Biomarkers of tumor-reactive CD4+ and CD8+ TILs associate with improved prognosis in endometrial cancer. J Immunother Cancer. 2022 Dec;10(12):e005443. doi: 10.1136/jitc-2022-005443. PMID: 36581331; PMCID: PMC9806064.
- Levy PL, Gros A. Fast track to personalized TCR T cell therapies. Cancer Cell. 2022 May 9;40(5):447-449.
- Gartner JJ, Parkhurst MR, Gros A, Tran E, Jafferji MS, Copeland A, Hanada KI, Zacharakis N, Lalani A, Krishna S, Sachs A, Prickett TD, Li YF, Florentin M, Kivitz S, Chatmon SC, Rosenberg SA, Robbins PF. A machine learning model for ranking candidate HLA class I neoantigens based on known neoepitopes from multiple human tumor types. Nat Cancer. 2021 May;2(5):563-574.
- Arenas EJ, Martínez-Sabadell A, Rius Ruiz I, Román Alonso M, Escorihuela M, Luque A, Fajardo CA, Gros A, Klein C, Arribas J. Acquired cancer cell resistance to T cell bispecific antibodies and CAR T targeting HER2 through JAK2 down-modulation. Nat Commun. 2021 Feb 23;12(1):1237.
- Kast F, Klein C, Umaña P, Gros A, Gasser S. Advances in identification and selection of personalized neoantigen/T-cell pairs for autologous adoptive T cell therapies. Oncoimmunology. 2021 Jan 7;10(1):1869389.
- Arenas EJ, Martínez-Sabadell A, Rius Ruiz I, Román Alonso M, Escorihuela M, Luque A, Fajardo CA, Gros A, Klein C, Arribas J. Acquired cancer cell resistance to T cell bispecific antibodies and CAR T targeting HER2 through JAK2 down-modulation. Nat Commun. 2021 Feb 23;12(1):1237. doi: 10.1038/s41467-021-21445-4. PMID: 33623012; PMCID: PMC7902842.
- Kast F, Klein C, Umaña P, Gros A, Gasser S. Advances in identification and selection of personalized neoantigen/T-cell pairs for autologous adoptive T cell therapies. Oncoimmunology. 2021 Jan 7;10(1):1869389. doi: 10.1080/2162402X.2020.1869389. PMID: 33520408; PMCID: PMC7808433.
- Lozano-Rabella M, Gros A. TCR Repertoire Changes during TIL Expansion: Clonal Selection or Drifting? Clin Cancer Res. 2020 Aug 15;26(16):4177-4179
- Gros A, Tran E, Parkhurst MR, Ilyas S, Pasetto A, Groh EM, Robbins PF, Yossef R, Garcia-Garijo A, Fajardo CA, Prickett TD, Jia L, Gartner JJ, Ray S, Ngo L, Wunderllich JR, Yang JC, Rosenberg SA. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J Clin Invest. 2019 Nov 1;129(11):4992-5004.
- Garcia-Garijo A, Fajardo CA, Gros A. Determinants for Neoantigen Identification. Front Immunol. 2019 Jun 24;10:1392.
- Yossef R, Tran E, Deniger DC, Gros A, Pasetto A, Parkhurst MR, Gartner JJ, Prickett TD, Cafri G, Robbins PF, Rosenberg SA. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight. 2018 Oct 4;3(19). pii: 122467.
- R. Eil, S.K. Vodnala, D. Clever, C.A. Klebanoff, M.Sukumar, J.H. Pan, D.C. Palmer, A. Gros, T.N. Yamamoto, S.J. Patel, G.C. Guittard, Z. Yu, V. Carbonaro, K. Okkenhaug, D.S. Schrump, W.M. Linehan, R. Roychoudhuri, N.P. Restifo. Ionic immune suppression within the tumour microenvironment limits T cell effector function, Nature, 14 (2016) 539-543.
- A. Pasetto, A. Gros, P.F. Robbins, D.C. Deniger, R.D. Prickett, R. Matus-Nicodemos, D.C. Douek, B. Howie, H. Robins, M.R. Parkhurst, J. Gartner, K. Trebska-McGowan, J.S. Crystal, S.A. Rosenberg.Tumor- and Neoantigen-reactive T-cell receptors can be identified based on their frequency in fresh tumor.Cancer Immunol 2 (2016) 734-743.
- T.D. Prickett, J.S. Crystal, C.J. Cohen, A. Pasetto, M.R. Parkhurst, J.J. Gartner, X. Yao, R. Wang, A. Gros, Y.F. Li, M. El-Gamil, K. Trebska-McGowan, S.A. Rosenberg, P.F. Robbins, Durable Complete Response from Metastatic Melanoma after Transfer of Autologous T Cells Recognizing 10 Mutated Tumor Antigens, Cancer Immunol Res, (2016).
- C.A. Klebanoff, C.D. Scott, A.J. Leonardi, T.N. Yamamoto, A.C. Cruz, C. Ouyang, M. Ramaswamy, R. Roychoudhuri, Y. Ji, R.L. Eil, M. Sukumar, J.G. Crompton, D.C. Palmer, Z.A. Borman, D. Clever, S.K. Thomas, S. Patel, Z. Yu, P. Muranski, H. Liu, E. Wang, F.M. Marincola, A. Gros, L. Gattinoni, S.A. Rosenberg, R.M. Siegel, N.P. Restifo, Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy, J Clin Invest, 126 (2016) 318-334.
- A. Gros, M.R. Parkhurst, E. Tran, A. Pasetto, P.F. Robbins, S. Ilyas, T.D. Prickett, J.J. Gartner, J.S. Crystal, I.M. Roberts, K. Trebska-McGowan, J.R. Wunderlich, J.C. Yang, S.A. Rosenberg, Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients, Nat Med, 22 (2016) 433-438.
- E. Tran, M. Ahmadzadeh, Y.C. Lu, A. Gros, S. Turcotte, P.F. Robbins, J.J. Gartner, Z. Zheng, Y.F. Li, S. Ray, J.R. Wunderlich, R.P. Somerville, S.A. Rosenberg, Immunogenicity of somatic mutations in human gastrointestinal cancers, Science, 350 (2015) 1387-1390.
- L.M. Draper, M.L. Kwong, A. Gros, S. Stevanovic, E. Tran, S. Kerkar, M. Raffeld, S.A. Rosenberg, C.S. Hinrichs, Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6, Clin Cancer Res, 21 (2015) 4431-4439.
- J.G. Crompton, M. Sukumar, R. Roychoudhuri, D. Clever, A. Gros, R.L. Eil, E. Tran, K. Hanada, Z. Yu, D.C. Palmer, S.P. Kerkar, R.D. Michalek, T. Upham, A. Leonardi, N. Acquavella, E. Wang, F.M. Marincola, L. Gattinoni, P. Muranski, M.S. Sundrud, C.A. Klebanoff, S.A. Rosenberg, D.T. Fearon, N.P. Restifo, Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics, Cancer Res, 75 (2015) 296-305.
- N. Acquavella, D. Clever, Z. Yu, M. Roelke-Parker, D.C. Palmer, L. Xi, H. Pflicke, Y. Ji, A. Gros, K. Hanada, I.S. Goldlust, G.U. Mehta, C.A. Klebanoff, J.G. Crompton, M. Sukumar, J.J. Morrow, Z. Franco, L. Gattinoni, H. Liu, E. Wang, F. Marincola, D.F. Stroncek, C.C. Lee, M. Raffeld, M.W. Bosenberg, R. Roychoudhuri, N.P. Restifo, Type I cytokines synergize with oncogene inhibition to induce tumor growth arrest, Cancer Immunol Res, 3 (2015) 37-47.
- S. Turcotte, A. Gros, E. Tran, C.C. Lee, J.R. Wunderlich, P.F. Robbins, S.A. Rosenberg, Tumor-Reactive CD8+ T Cells in Metastatic Gastrointestinal Cancer Refractory to Chemotherapy, Clin Cancer Res, 20 (2014) 331-343.
- E. Tran, S. Turcotte, A. Gros, P.F. Robbins, Y.C. Lu, M.E. Dudley, J.R. Wunderlich, R.P. Somerville, K. Hogan, C.S. Hinrichs, M.R. Parkhurst, J.C. Yang, S.A. Rosenberg, Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer, Science, 344 (2014) 641-645.
- A. Gros, P.F. Robbins, X. Yao, Y.F. Li, S. Turcotte, E. Tran, J.R. Wunderlich, A. Mixon, S. Farid, M.E. Dudley, K. Hanada, J.R. Almeida, S. Darko, D.C. Douek, J.C. Yang, S.A. Rosenberg, PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors, J Clin Invest, 124 (2014) 2246-2259.
- S. Turcotte, A. Gros, K. Hogan, E. Tran, C.S. Hinrichs, J.R. Wunderlich, M.E. Dudley, S.A. Rosenberg, Phenotype and function of T cells infiltrating visceral metastases from gastrointestinal cancers and melanoma: implications for adoptive cell transfer therapy, J Immunol, 191 (2013) 2217-2225.
- R.A. Morgan, N. Chinnasamy, D. Abate-Daga, A. Gros, P.F. Robbins, Z. Zheng, M.E. Dudley, S.A. Feldman, J.C. Yang, R.M. Sherry, G.Q. Phan, M.S. Hughes, U.S. Kammula, A.D. Miller, C.J. Hessman, A.A. Stewart, N.P. Restifo, M.M. Quezado, M. Alimchandani, A.Z. Rosenberg, A. Nath, T. Wang, B. Bielekova, S.C. Wuest, N. Akula, F.J. McMahon, S. Wilde, B. Mosetter, D.J. Schendel, C.M. Laurencot, S.A. Rosenberg, Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy, J Immunother, 36 (2013) 133-151.
- C. Puig-Saus, A. Gros, R. Alemany, M. Cascallo, Adenovirus i-leader truncation bioselected against cancer-associated fibroblasts to overcome tumor stromal barriers, Mol Ther, 20 (2012) 54-62.
- S. Guedan, D. Grases, J.J. Rojas, A. Gros, F. Vilardell, R. Vile, E. Mercade, M. Cascallo, R. Alemany, GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution, Gene Ther, 19 (2012) 1048-1057.
- A. Gros, S. Turcotte, J.R. Wunderlich, M. Ahmadzadeh, M.E. Dudley, S.A. Rosenberg, Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma, Clin Cancer Res, 18 (2012) 5212-5223.
- M. Gimenez-Alejandre, A. Gros, R. Alemany, Construction of capsid-modified adenoviruses by recombination in yeast and purification by iodixanol-gradient, Methods Mol Biol, 797 (2012) 21-34.
- L. Coughlan, S. Vallath, A. Gros, M. Gimenez-Alejandre, N. Van Rooijen, G.J. Thomas, A.H. Baker, M. Cascallo, R. Alemany, I.R. Hart, Combined fiber modifications both to target alpha(v)beta(6) and detarget the coxsackievirus-adenovirus receptor improve virus toxicity profiles in vivo but fail to improve antitumoral efficacy relative to adenovirus serotype 5, Hum Gene Ther, 23 (2012) 960-979.
- S. Guedan, J.J. Rojas, A. Gros, E. Mercade, M. Cascallo, R. Alemany, Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth, Mol Ther, 18 (2010) 1275-1283.
- A. Gros, C. Puig, S. Guedan, J.J. Rojas, R. Alemany, M. Cascallo, Verapamil enhances the antitumoral efficacy of oncolytic adenoviruses, Mol Ther, 18 (2010) 903-911.
- 22.- J.J. Rojas, M. Cascallo, S. Guedan, A. Gros, J. Martinez-Quintanilla, A. Hemminki, R. Alemany, A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses, Gene Ther, 16 (2009) 1441-1451.
- J. Martinez-Quintanilla, M. Cascallo, A. Gros, C. Fillat, R. Alemany, Positive selection of gene-modified cells increases the efficacy of pancreatic cancer suicide gene therapy, Mol Cancer Ther, 8 (2009) 3098-3107.
- M. Huch, A. Gros, A. Jose, J.R. Gonzalez, R. Alemany, C. Fillat, Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models, Neoplasia, 11 (2009) 518-528, 514 p following 528.
- N. Bayo-Puxan, M. Gimenez-Alejandre, S. Lavilla-Alonso, A. Gros, M. Cascallo, A. Hemminki, R. Alemany, Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting, Hum Gene Ther, 20 (2009) 1214-1221.
- S. Guedan, A. Gros, M. Cascallo, R. Vile, E. Mercade, R. Alemany, Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication, Gene Ther, 15 (2008) 1240-1245.
- A. Gros, J. Martinez-Quintanilla, C. Puig, S. Guedan, D.G. Mollevi, R. Alemany, M. Cascallo, Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency, Cancer Res, 68 (2008) 8928-8937.
- M. Cascallo, A. Gros, N. Bayo, T. Serrano, G. Capella, R. Alemany, Deletion of VAI and VAII RNA genes in the design of oncolytic adenoviruses, Hum Gene Ther, 17 (2006) 929-940.
- N. Bayo-Puxan, M. Cascallo, A. Gros, M. Huch, C. Fillat, R. Alemany, Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting, J Gen Virol, 87 (2006) 2487-2495.
- Grantor: Instituto de Salud Carlos III (ISCIII).
Title: Terapia celular de próxima generación con TIL específicos de neoantígenos para pacientes con tumores resistentes a inhibidores de puntos de control inmunitario. - Grantor: Fundación Fero (Beca Fero).
Title: Non-invasive personalized T-cell therapies targeting recurrent hot spot driver mutations in cancer. - Grantor: Fundación BBVA.
Alena Gros is a member of the VHIO-BBVA Foundation Comprehensive Program of Cancer Immunotherapy & Immunology (CAIMI). - Grantor: La Marató de TV3.
Title: Personalized Immunotherapy for Endometrial Cancer. - Grantor: La Caixa Health Research.
Title: Development of enabling technologies for T-cell immunotherapy of solid tumors. - Grantor: Ministerio de Ciencia e Innovación. Title: Mining the molecular determinants of the personalized T-cell response in cancer patients to develop more effective immunotherapies.
- Title: Personalized T-cell therapies targeting neoantigens with enhanced efficacy and broad applicability (PersACT)
Reference: CNS2023-145343
Funding Entity: Agencia Estatal de Investigación. Ministerio de Ciencia e Innovación
Period of Execution: 1/04/2024-31/03/2026
PI: Alena Gros
Ayuda cofinanciada por la Unión Europea Next Generation EU – Plan de Recuperación Transformación y Resilencia
![]()















