
Established in 1997, our Clinical Trials Office incorporates experts conducting clinical trials at the Vall d’Hebron University Hospital’s (HUVH) Medical Oncology Department, headed by VHIO’s Director Josep Tabernero.
This Office comprises study coordinators, data managers and administrative staff who coordinate phase I–IV clinical trials and also participate in several translational research projects conducted at VHIO. Our team, managed by the Clinical Trials Office Director, Cristina Pérez, is organized into 4 groups; start-up unit, oncology study coordinators, oncology data entries, hematology study coordinators and data entries, covering all tumor types and clinical trials.
In oncology and hematology, in 2023 we managed a total of 276 phase I trials, 29 basket trials, 183 phase II trials, 190 phase III clinical trials, and 3 medical device trials and 81 post-authorization and rollover studies, with active recruitment at some time in the year, and patient enrolment in our oncology and hematology clinical trials totaled at 1,343. In addition, we managed 1 phase II trial, 3 phase III trials and 1 post-authorization study in radiotherapy including a total of 14 patients and we continue to follow up patients who were recruited prior to 2023 and are still enrolled and receiving study treatment (5 patients in total, and 38 in follow-up) in Phase II and III trials.
203 new trials were initiated in 2023, including 22 post-authorization trials and rollover studies. In addition, we continue to follow up patients who were recruited prior to 2023 and are still enrolled and receiving study treatment (1,105 patients in total, and 2,001 in follow-up) in Phase 0, I, II, III and medical device trials.

Cristina Pérez
Head of the CTO
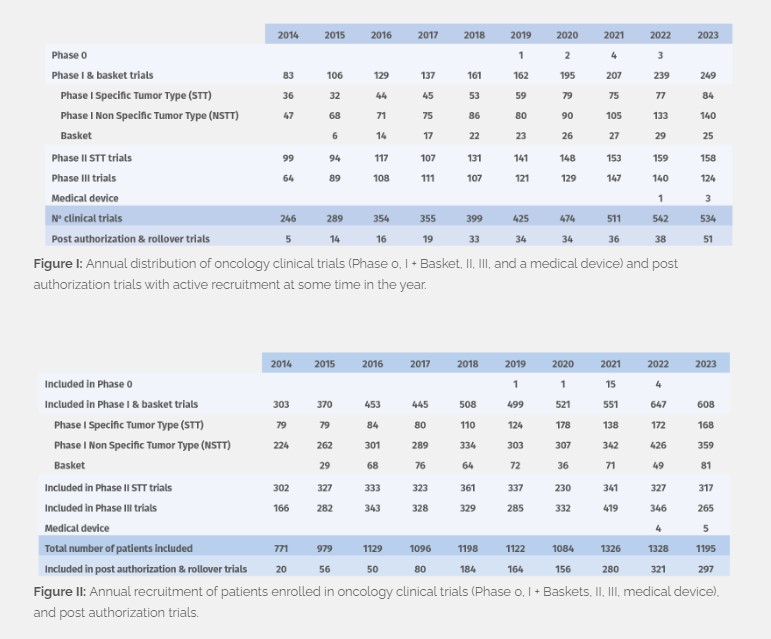
In 2023 we managed 224 phase I, 25 basket, 158 phase II, and 124 phase III clinical trials, and 3 medical device trials with active recruitment at some time in the year (Figure I), with patient enrolment totaling at 1,195 (Figure II). 162 new trials were initiated, including 15 post-authorization trials and rollover studies. In addition, we continue to follow up patients who were recruited prior to 2023 and are still enrolled and receiving study treatment (940 patients in total, and 1,776 in follow- up) in Phase 0, I, II, III and medical device trials.
More than half of our patients included in our phase I clinical trials have been referred to us from other hospitals, which has consequently positioned our Unit as a leading reference in early clinical studies. Reflective of our recognized excellence, VHIO’s Research Unit for Molecular Therapy of Cancer (UITM) – CaixaResearch, directed by Elena Garralda, has been re-accredited by the Generalitat de Catalunya (Government of Catalonia).
As we continue to render personalized medicine more precise by matching therapies to the specificities of each individual patient, the requirements and selection criteria for inclusion in certain studies are becoming more complex.
We are dedicated to expanding our portfolio of trials to ultimately establish new treatment regimens with highly selective drugs. Our Unit continues to fine-tune patient selection criteria to identify those patients who are most likely to benefit from novel therapies, including emerging immune-based treatments, tailored to individual patients’ molecular features.
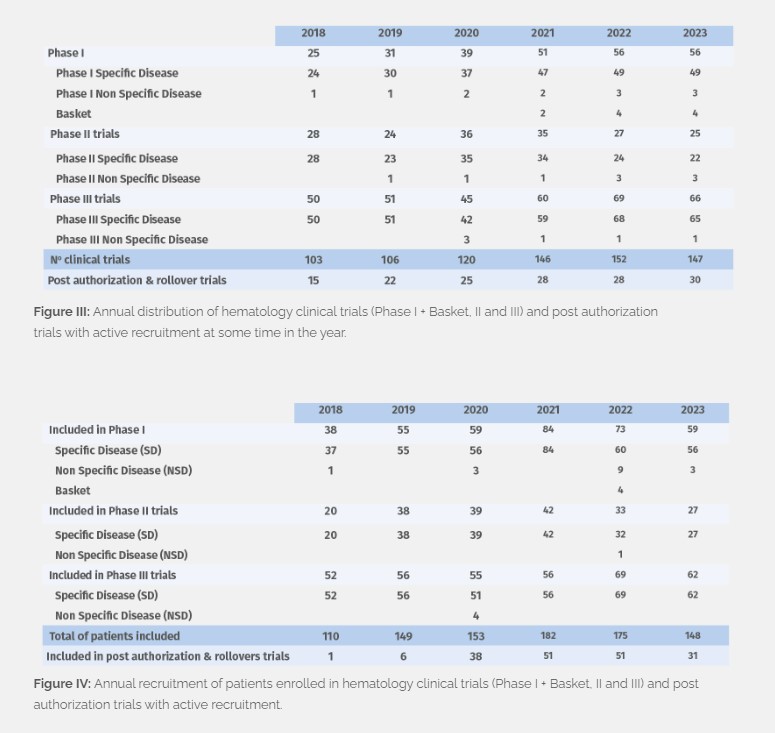
In 2023 we managed 52 phase I, 4 basket, 25 phase II and 66 phase III clinical studies with active recruitment at some time in the year (Figure III), with patient enrollment totaling at 148 (Figure IV). 40 new trials were initiated, including 7 post-authorization trials and rollover studies. In addition, we continue to follow up patients who were recruited prior to 2023 and are still enrolled and receiving study treatment (160 patients in total, and 187 in follow-up) in Phase I, II and III trials.
Clinical research is hematology is spearhead by Francesc Bosch, Principal Investigator of VHIO’s Experimental Hematology Group.
The prestige of HUVH’s Medical Oncology Department is recognized by pharmaceutical and biotechnology companies. It has also become a reference program and selected by the industry to carry out complex clinical trials. The number of participating centers in these studies is highly restricted.
Clinical sites are selected based on the highest quality standards and capacity for carrying out state-of-the-art research. We have participated in early phase trials of different drugs, ultimately enabling the pharmaceutical industry to market novel anti-cancer medicines. We are involved in studies promoted by the pharmaceutical industry as well as those developed by us in collaboration with other hospitals. In 2023, we have also collaborated in about 16 investigator-Initiated trials (IITs) in oncology.
- Contribute to the development of novel therapies against cancer.
- Consolidation as an international reference for clinical trials in oncology and hematology.
- Guide patients enrolled in clinical trials to comply with the protocol requirements and help them with daily life throughout the duration of their participation.
- Standardize clinical trial processes to ensure optimal quality and the compliance of Good Clinical Practice (GCP).
- To incorporate novel tools and procedures to improve the quality of the CTs conducted in our center.
- To reduce data entry time and implement a remote monitoring system.
- To establish standard operating procedures for the launch of novel CTs.
- To collaborate with Sponsors and CROs for the implementation of platforms that facilitate the launch of CTs and storage and updating of the associated documentation.
- To provide individualized training to our personnel to improve their performance and professional skills and competences.
Clinical Trials Office Director
Cristina Pérez
Head, Start-Up Unit and Clinical Trials
Liaison Ana Matres
Coordinator, Start-Up Unit
Nuria Farràs
Head of data entry
Ignacio Carcela
Head of Hematology
Laura Segura
Lead Study Coordinators
Eulalia Aliende, Enric Álvarez, Eva Banús, Queralt Ferrer, Montse Moreno, Gemma Mur, Olga Padrós, Julia Sedó
Lead Data entry
Gloria García, Eva Lázaro, Joana Pinyol, Alberto Rojo
Study Coordinators
Aitana Almodóvar, Gisela Andrés, Marina Barbero, Jorge Bardina, Laura Blanco, Anna Cabrera, Julia Caparrós, Ana Carmona, Laia Catalán, Paula Chiquillo, Natàlia Écija, Carlos Fernández, Danis Fernández, Alba Galiana, Anna Giralt, Carolina Gallo, Alexia García, Isaac Gímenez, Laia Gispert, Sara Gutiérrez, Sara Herbera, Montserrat Hernández, Marta Horcas, Silvia Hurtado, Josu Iraola, Bàrbara Juanmiquel, Alejandro Lahire, Esther Llaudet, Raquel Madrenas, Marc Majó, Sonia Martínez, Magda Masana, Alba Meire, Mireia Mira, Thaís Miquel, Anna Peñalver, Montserrat Pequera, Jordi Perera, Gemma Pujadas, Cristian Rosales, Marta Rotxés, Álvaro Rueda, Laura Sancho, Laura Saucedo, Samira Sehir, Albert Teixidor, Julia Toledo
Data Entries
Cristina Aguilar, David Alvarez, Maria Ayala, Nestor Babon, Aitor Bañon, Maria Barber, Samanta Bascuas, Carlota Bellot, Laia Benitez, Cristina Calderon, Helena Carbonero, Anna Cos, Ruben Díaz, Nora Dieguez, Andrea Fores, Marta Garcia, Mariona Guillamet, Andrea Illescas, Elisa Irles, Neus Iserte, Sandra Justicia, Julia Laborda, Eva Marín, Sílvia Marín, Mireia Masot, Carina Monclús, Andrea Monzó, Cristina Navarro, Ana Nicolas, Paula Nuñez, Maria Ortega, Laura Pallares, Sergio Perez, Xavier Perez, Eva Puerma, Isabel Rico, Maria Rion, Angel Romano, Jordi Romero, Rosa Romero, Rebeca Sanchez, Lucia Sanchis, Judith Serrano, Hugo Somoza, Marta Urdi, Marta Vidigal, Laia Vila
Clinical Trials Assistants
Cristian Campderros, Juan López, Alicia Nogueroles, Sara Vázquez











