
Sarcoma encompasses >70 subtypes of mesenchymal origin, constituting 1-2% of all cancers. From a biological perspective, sarcomas can be classified into two broad categories: sarcomas driven by simple genetic alterations, such as translocations or specific activating mutations; and those with complex and unbalanced genomic profiles. Both include a broad diversity of entities, often with very different molecular characteristics, course of disease and therapeutic implications. Our group focuses on sarcoma translational research, with an emphasis on biological understanding of sarcomas oriented to biomarker and drug development.
Among these, gastrointestinal stromal tumors (GISTs) represent the most common malignant mesenchymal neoplasm and constitutes a paradigm model for studying oncogene addiction and identifying structural and functional mechanisms of response and resistance to treatment. In recent years, our work has advanced the understanding of the pathophysiological mechanisms of GIST and exposed critical insights on the complex evolution of KIT/PDGFRA driver mutations leading to therapy resistance through comprehensive analyses of tissue and liquid biopsy samples. Currently ongoing efforts on GIST include: 1) The GISTomics project—a European initiative led by our group—aims to advance insights into the landscape of GIST evolution; and 2) A real-world-based genomic and transcriptomic landscape in a cohort of 1,427 GIST cases, the largest series available to date.
As part of our translational efforts, we pursue the identification of critical molecular mediators of therapeutic adaptation and resistance in GIST. Our work involves the discovery and functional characterization of key molecular events that can be potentially targetable. These mediators range from signaling intermediates, to the unfolded protein response (UPR) and interactions with the tissue microenvironment (TME), all of which are involved in the sensitivity/ resistance to KIT inhibition. In this sense, César Serrano’s twin lab at the Institute for Research in Biomedicine (IRB), within the TRIP Clinics program, expands these views to the fields of protein degradation and the ubiquitin-proteasome system. Additionally, we leverage multi-omic datasets to identify and characterize the patterns and function of chromosome instability in sarcoma progression.
Our aim is to have a true clinical impact by improving the daily treatment and care of our patients. We are proud that our Sarcoma Multidisciplinary Unit is a Spanish Health Ministry-designed referral center (CSUR), and we belong to the European Reference Network ERN-EURACAN, which therefore constitutes an optimal setting for translating cancer discovery into clinical benefits. In this sense, three highlights from 2024 include the finalization of the MOTION trial, that confirmed the activity of vimseltinib in patients with tenosynovial giant cell tumor (TGCT) (Gelderblom et al, Lancet 2024); the launching of the phase III INSIGHT trial, which aim to validate in the clinic the use of ctDNA to guide therapeutic decisions in GIST (NCT05734105); the involvement in the updated guidelines from the European Society of Medical Oncology (ESMO) to establish the role of next-generation sequencing (NGS) in cancer, including sarcomas and other rare tumors.
- Identification of critical molecular mediators of oncogenic signaling in sarcomas.
- Studying the patterns of CIN in sarcomas together with its role in tumor evolution and progression.
- Characterization of mechanisms of response and resistance to targeted therapies in sarcomas, including preclinical modelling and validation of therapeutic strategies for their translation to the clinic.
- Phase I to phase III studies for clinical drug development in sarcomas.
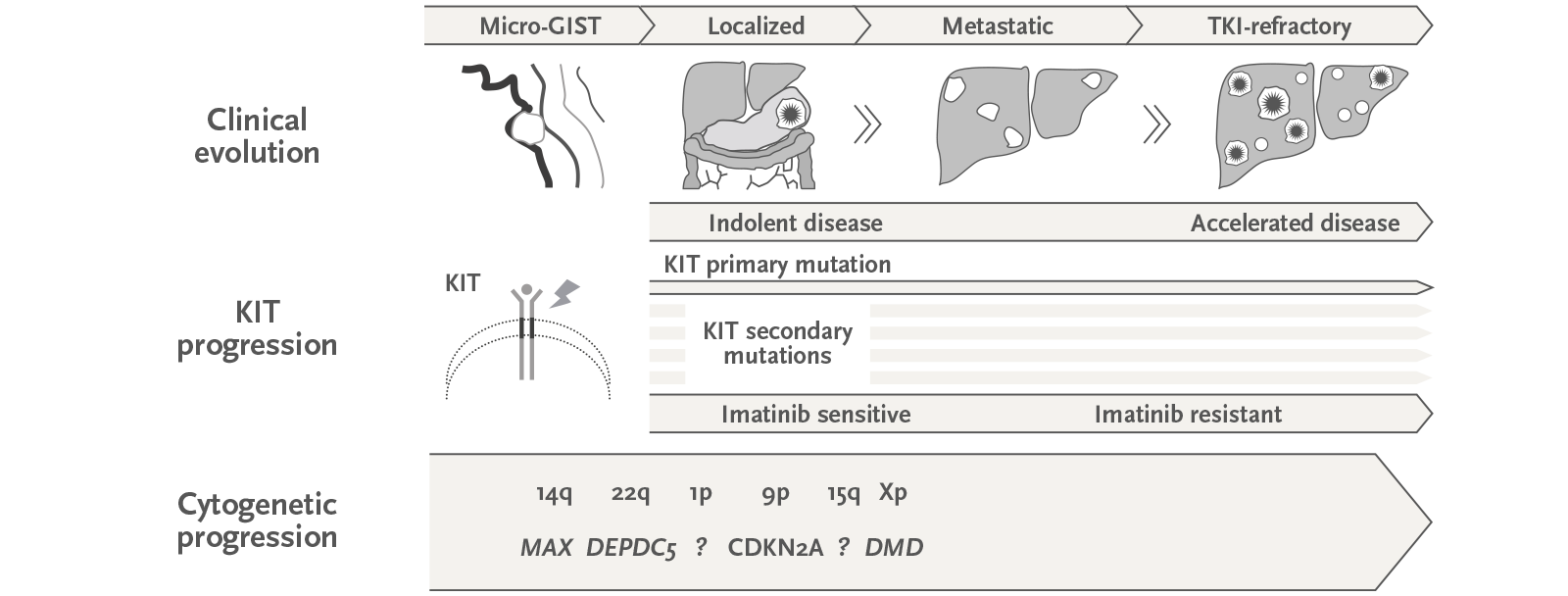
Figure: Gastrointestinal stromal tumor (GIST) is the most common malignant mesenchymal tumor and a successful and paradigmatic model to dissect mechanisms of response and resistance to molecularly targeted agents. Ongoing research from our group is advancing this knowledge in two directions: first, we are interrogating mutation-based mechanisms affecting the sensitivity to approved KIT inhibitors.
Second, we are investigating the cytogenetic progression that fuels GIST growth. These findings have a direct impact on the future of drug development in GIST and other diseases (Figure from Serrano & George, Clin Cancer Res 2020; 26: 923-934.).
Group Leader
César Serrano
Oncologists
Carlo M. Cicala
Davide Romandini
Post-Doctoral Fellow
Pawel Sobczuk
Pre-Doctoral Fellows
Jon Ander Aguirre Carrillo
Marc Arbonés
Júlia Caparrós Mateos
David Gómez Peregrina
Yulia Kremlyakova
Gemma Mur Bonet
Iván Olivares Rivas
Tulio Silva
Senior Technician
Jordi Rosell Aluja
Graduate Student
Laia Conchillo Puigmolé
Visiting Student
Francesca Esposito
Most Relevant Scientific Publications
- Ripretinib versus sunitinib in gastrointestinal stromal tumor: ctDNA biomarker analysis of the phase 3 INTRIGUE trial. Heinrich MC, Jones RL, George S, Gelderblom H, Schöffski P, von Mehren M, Zalcberg JR, Kang YK, Razak AA, Trent J, Attia S, Le Cesne A, Siontis BL, Goldstein D, Boye K, Sanchez C, Steeghs N, Rutkowski P, Druta M, Serrano C, Somaiah N, Chi P, Reichmann W, Sprott K, Achour H, Sherman ML, Ruiz-Soto R, Blay JY, Bauer S. Nat Med. 2024;30:498-506.
- KIT ATP-Binding Pocket/Activation Loop Mutations in GI Stromal Tumor: Emerging Mechanisms of Kinase Inhibitor Escape. Mühlenberg T, Falkenhorst J, Schulz T, Fletcher BS, Teuber A, Krzeciesa D, Klooster I, Lundberg M, Wilson L, Lategahn J, von Mehren M, Grunewald S, Tüns AI, Wardelmann E, Sicklick JK, Brahmi M, Serrano C, Schildhaus HU, Sievers S, Treckmann J, Heinrich MC, Raut CP, Ou WB, Marino-Enriquez A, George S, Rauh D, Fletcher JA, Bauer S. J Clin Oncol. 2024;42:1439-1449.
- Serrano C, Rothschild S, Villacampa G, Heinrich MC, George S, Blay JY, Sicklick JK, Schwartz GK, Rastogi S, Jones RL, Rutkowski P, Somaiah N, Navarro V, Evans D, Trent JC. Rethinking placebos: embracing synthetic control arms in clinical trials for rare tumors. Nat Med. 2023 Nov;29(11):2689-2692.
- Serrano C, Bauer S, Gómez-Peregrina D, Kang YK, Jones RL, Rutkowski P, Mir O, Heinrich MC, Tap WD, Newberry K, Grassian A, Shi H, Bialick S, Schöffski P, Pantaleo MA, von Mehren M, Trent JC, George S. Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib. Ann Oncol. 2023 Jul;34(7):615-625.
- García-Valverde A, Rosell J, Sayols S, Gómez-Peregrina D, Pilco-Janeta DF, Olivares-Rivas I, de Álava E, Maurel J, Rubió-Casadevall J, Esteve A, Gut M, Valverde C, Barretina J, Carles J, Demetri GD, Fletcher JA, Arribas J, Serrano C. E3 ubiquitin ligase Atrogin-1 mediates adaptive resistance to KIT-targeted inhibition in gastrointestinal stromal tumor. Oncogene. 2021 Dec;40(48):6614-6626.
- Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, Schöffski P, Jones RL, Attia S, D’Amato G, Chi P, Reichardt P, Meade J, Shi K, Ruiz-Soto R, George S, von Mehren M. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a doubleblind, randomised, placebocontrolled, phase 3 trial. Lancet Oncol. 2020 Jul;21(7):923-934.
- Heinrich MC, Jones RL, von Mehren M, Schöffski P, Serrano C, Kang YK, Cassier PA, Mir O, Eskens F, Tap WD, Rutkowski P, Chawla SP, Trent J, Tugnait M, Evans EK, Lauz T, Zhou T, Roche M, Wolf BB, Bauer S, George S. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020 Jul;21(7):935-946.
- Serrano C, Leal A, Kuang Y, Morgan JA, Barysauskas CM, Phallen J, Triplett O, Mariño-Enríquez A, Wagner AJ, Demetri GD, Velculescu VE, Paweletz CP, Fletcher JA, George S. Phase I Study of Rapid Alternation of Sunitinib and Regorafenib for the Treatment of Tyrosine Kinase Inhibitor Refractory Gastrointestinal Stromal Tumors. Clin Cancer Res. 2019 Dec 15;25(24):7287-7293.
- Serrano C, Mariño-Enríquez A, Tao DL, Ketzer J, Eilers G, Zhu M, Yu C, Mannan AM, Rubin BP, Demetri GD, Raut CP, Presnell A, McKinley A, Heinrich MC, Czaplinski JT, Sicinska E, Bauer S, George S, Fletcher JA. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019 Mar;120(6):612-620.
- Serrano C, Wang Y, Mariño-Enríquez A, Lee JC, Ravegnini G, Morgan JA, Bertagnolli MM, Beadling C, Demetri GD, Corless CL, Heinrich MC, Fletcher JA. KRAS and KIT Gatekeeper Mutations Confer Polyclonal Primary Imatinib Resistance in GI Stromal Tumors: Relevance of Concomitant Phosphatidylinositol 3-Kinase/AKT Dysregulation. J Clin Oncol. 2015 Aug 1;33(22):e93-6.
- Determinantes clínicos y moleculares de respuesta y progresión a la inhibición de KIT en pacientes con tumores del estroma gastrointestinal (GIST) con respuesta prolongada a Imatinib. Grupo Español de Investigación en Sarcomas (GEIS), Sociedad Española de Oncología Médica (SEOM), and Fundación Mari Paz Jiménez Casado.
- Modulating our ubiquitination machinery to innovate treatments and drug discovery in cancer. Asociación Española Contra el Cáncer– AECC (Spanish Association against Cancer).
- Perfiles de resistencia molecular a la inhibición de KIT/PDGFRA en tumores del estroma gastrointestinal. Asociación Española Contra el Cáncer – AECC (Spanish Association against Cancer).
- Centralization of pathological diagnosis and implementation of precision medicine strategies in sarcomas. Asociación Española Contra el Cáncer – AECC (Spanish Association against Cancer).
- Genomic determinants of tumor evolution and progression in sarcomas with simple karyotype. CRIS Foundation.
- Drivers of chromosome instability in karyotypically simple sarcomas: gastrointestinal stromal tumor as a paradigm. Instituto de Salud Carlos III.
- Yin-yang modulation of the ubiquitin-proteasome system to chart and treat RTK-driven neoplasms. TRIP-Clinics program (La Caixa Foundation and Generalitat de Catalunya).
The Sarcoma Translational Research Group receives support from the FERO Foundation through a Start-Up grant for the creation and consolidation of the group in the VHIO.























