- Published in the journal Genes & Development*, results of a study led by Laura Soucek, co-Director of the Vall d’Hebron Institute of Oncology’s Preclinical and Translational Research Program, show that the expression of Omomyc in preclinical melanoma models disrupts MYC activity and alters gene expression profiles, reducing cancer proliferation and progression.
- Results suggest that Omomyc MYC-targeted therapy could potentially open up a new treatment avenue for melanoma, and point to the future development of clinical trials to assess the efficacy of this mighty mini-protein against this tumor type.
- Developed by VHIO-born spin-off Peptomyc, Omomyc is the most cheracterized MYC inhibitor to date. This novel targeted therapy has successfully completed a phase I clinical trial.
Cutaneous melanoma, the most aggressive form of skin cancer, is the fifth most diagnosed cancer in Europe and one of the most frequent causes of cancer death, with incidence and mortality trends on the rise. While the development of more effective treatments including immune-based approaches have improved survival in some patients, many do not benefit from these anti-cancer therapies due to intrinsic or acquired drug resistance.
“In 2020, over 105,000 people were diagnosed with cutaneous melanoma and 16,000 died of this disease in the EU. Rising incidence and mortality rates also illuminate the urgent need to develop new, more effective therapies against this tumor type,” says Laura Soucek, co-Director of the Vall d’Hebron Institute of Oncology’s (VHIO) Preclinical and Translational Research Program, and Principal Investigator of our Models of Cancer Therapies Group.
Built on over 20 years of research led by Laura Soucek, the Omomyc (OMO-103) therapeutic mini-protein was developed in-house by the VHIO and Catalan Institute of Research and Advanced Studies’ (ICREA) spin-off company Peptomyc S.L., which she co-founded in 2014. Having previously shown the preclinical efficacy and safety of this novel cell-penetrating mini-protein in mouse models, her team successfully developed anti-MYC peptides for the treatment of several tumor types.
Omomyc is showing increasing promise in becoming the first ever clinically viable and direct inhibitor of the MYC oncogene — a transcription factor and major driver of multiple tumor types. This novel MYC inhibitor has now successfully completed a phase I clinical trial.
Published in the journal Genes & Development*, results of a study directed by Soucek now demonstrate the preclinical promise of Omomyc in opening up a new treatment avenue for patients with melanoma.
“The deregulation of MYC, occurring in up to 70% of human cancers, induces the transcription of genes implicated in myriad processes including the development of metastases and disease recurrence. The overexpression of this protein is found in 34% of melanomas and associates with increased aggressiveness of disease and several pathways of treatment resistance. MYC inhibition could therefore promise an important therapeutic impact against this disease,” observes Soucek, CEO of Peptomyc, an ICREA Research Professor and co-corresponding author of this study.
“Preclinical data from this present study show that reducing MYC’s transcriptional footprint by the transgenic expression of Omomyc, achieves a significant reduction in tumor growth and metastatic capacity, independently of the driver mutation,” adds Mariano F. Zacarías-Fluck, Research Associate of VHIO’s Models of Cancer Therapies Group and co-corresponding author of this study.
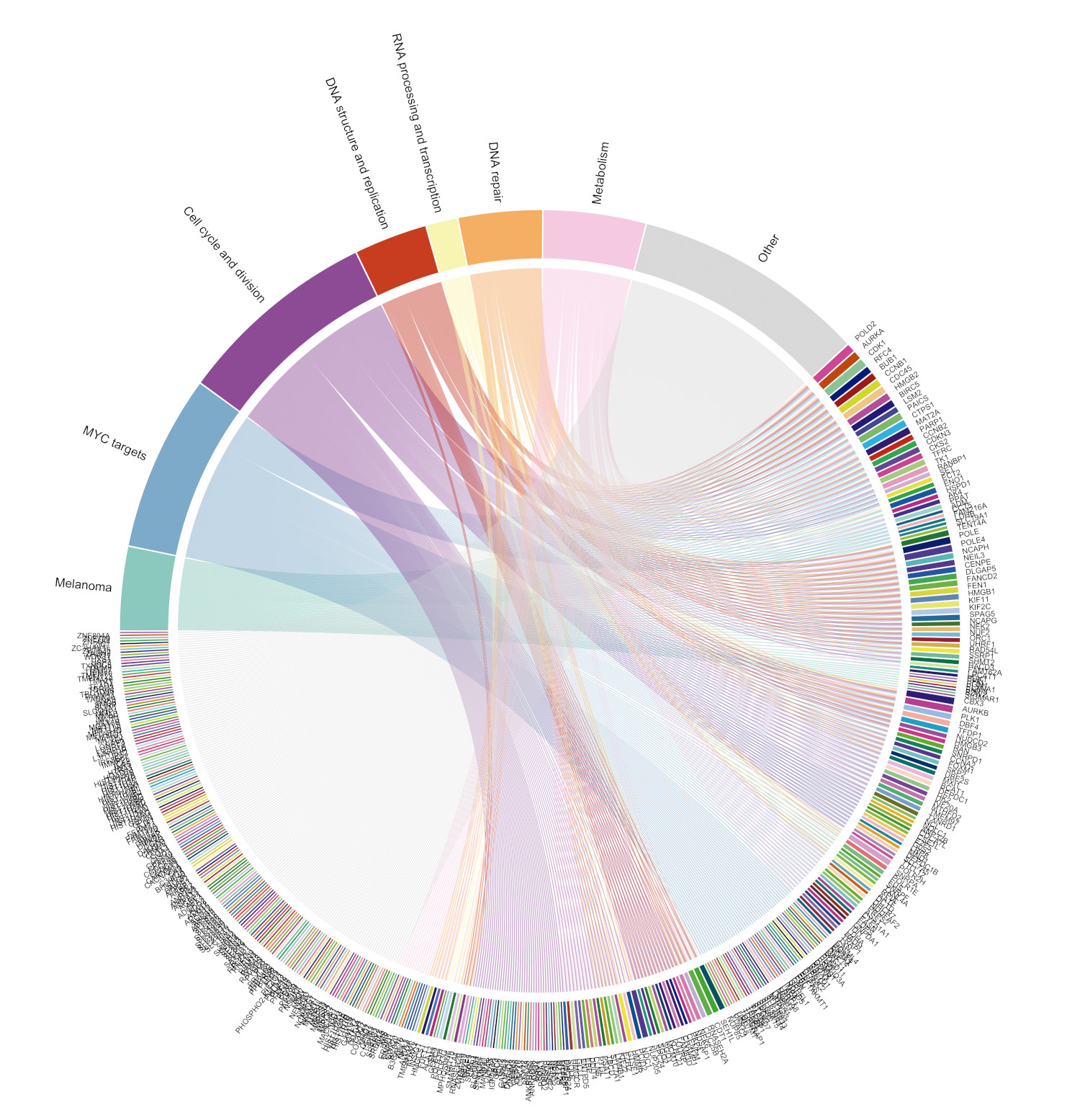
Omomyc-induced transcriptional modulation independent of melanoma driver mutation targets key melanoma genes. Genes downregulated by Omomyc belong to categories relevant to melanoma and MYC biology. This figure has been chosen by Genes&Development magazine as the cover image of its next issue on paper
The investigators report that MYC inhibitions halts the transcription of genes implicated in cell growth and proliferation, and that the gene expression profiles underlying aggressive disease return to similar profiles observed in melanoma patients with a good prognosis.
They initially conducted this study in vitro in 9 melanoma cell lines with different types of mutations. Omomyc expression in tumor cells reduced MYC levels and significantly reduced cell proliferation in all lines, independently of the tumor mutation profile mutational profile, and also induced tumor cell death.
Results in vitro led them to study the efficacy of Omomyc in two mouse models of two highly aggressive types of melanoma. “Taken together, the results of the mouse model study show that Omomyc reduces melanoma cell proliferation, achieves tumor regression in vivo, decreases metastatic capacity, and prevents disease recurrence after the surgical resection of primary tumors in mice,” says Zacarías-Fluck.
“Our preclinical findings suggest that Omomyc’s inhibition of MYC could potentially improve outcomes in patients with melanoma, although this will need to be evaluated in future clinical trials,” concludes Soucek.
This research was supported through grants received from the Spanish Ministry of Economy and Competitiveness, Fundació La Marató de TV3, Spanish Ministry of Science and Innovation, Generalitat de Catalunya, and the European Research Council.
###
Reference:
Zacarías-Fluck MF, Massó-Vallés D, Giuntini F, González-Larreategui Í, Kaur J, Casacuberta-Serra S, Jauset T, Martínez-Martín S, Martín-Fernández G, Serrano Del Pozo E, Foradada L, Grueso J, Nonell L, Beaulieu ME, Whitfield JR, Soucek L. Reducing MYC’s transcriptional footprint unveils a good prognostic gene signature in melanoma. Genes Dev. 2023 Apr 6. doi: 10.1101/gad.350078.122. Epub ahead of print. PMID: 37024284.