

VHIO’s Molecular Prescreening Program, driven by FERO’s Institutional Advanced Molecular Diagnostics Program – DIAMAV, catalyzes precision medicine at VHIO. Over the last decade, this program has provided access to advanced molecular diagnostics to more than 9,000 cancer patients, and is critical in matching targeted therapeutic approaches with hundreds of clinical trial opportunities.
This program, also counting on the support and expertise provided through our Research Unit for Molecular Therapy of Cancer (UITM) – CaixaResearch, is co-led by VHIO’s Ana Vivancos, Paolo Nuciforo, Elena Garralda (also Director of the UITM), and Rodrigo Dienstmann, Principal Investigators of our Cancer Genomics, Molecular Oncology, Early Clinical Drug Development, and Oncology Data Science – OdysSey Groups, respectively. Activities are coordinated by Susana Aguilar, Head of the VHIOTECA Unit, in collaboration with Jenifer González, Research Support Technician (VHIO’s Cancer Genomics Group).
The main objective of molecular prescreening at VHIO is to facilitate the clinical implementation of emerging cancer biomarkers that help to optimize the selection of therapies for patients being considered for enrollment in clinical trials. Our program guides clinicians in selecting both standard-of-care and investigational anti-cancer treatments and spurs clinical-molecular correlative research at VHIO. Diagnostic tests are developed and validated in-house for the cost-effective and streamlined identification of tumor molecular alterations of major interest in drug development.
Tumor profiling includes a variety of genomic techniques including next-generation sequencing panels (NGS) for the detection of mutations, copy number variations, gene fusions and RNA expression signatures, as well as histopathological techniques such as immunohistochemistry (IHC) and in situ hybridization (ISH) for protein and gene expression profiling.
In 2023, we performed tumor molecular profiling of 1,968 cancer patients’ tumors, representing a significant increase compared to previous years. These patients are candidates for enrolment in clinical trials. Notably, since 2022 an NGS test has been implemented for the liquid biopsy detection of genomic alterations. This new test has been applied to 991 patients who may have acquired resistance to targeted therapies or patients without metatastic tumor tissue biopsies for testing.
Interpretation of next-generation sequencing tests and educating clinicians on emerging biomarkers is another of our priority areas. During Molecular Tumor Board and Genetic Tumor Board meetings, we facilitate data exchange among a broad range of experts for the review of patients’ medical histories and cancer molecular profiles in order to more precisely guide treatment decisions and preventive measures.
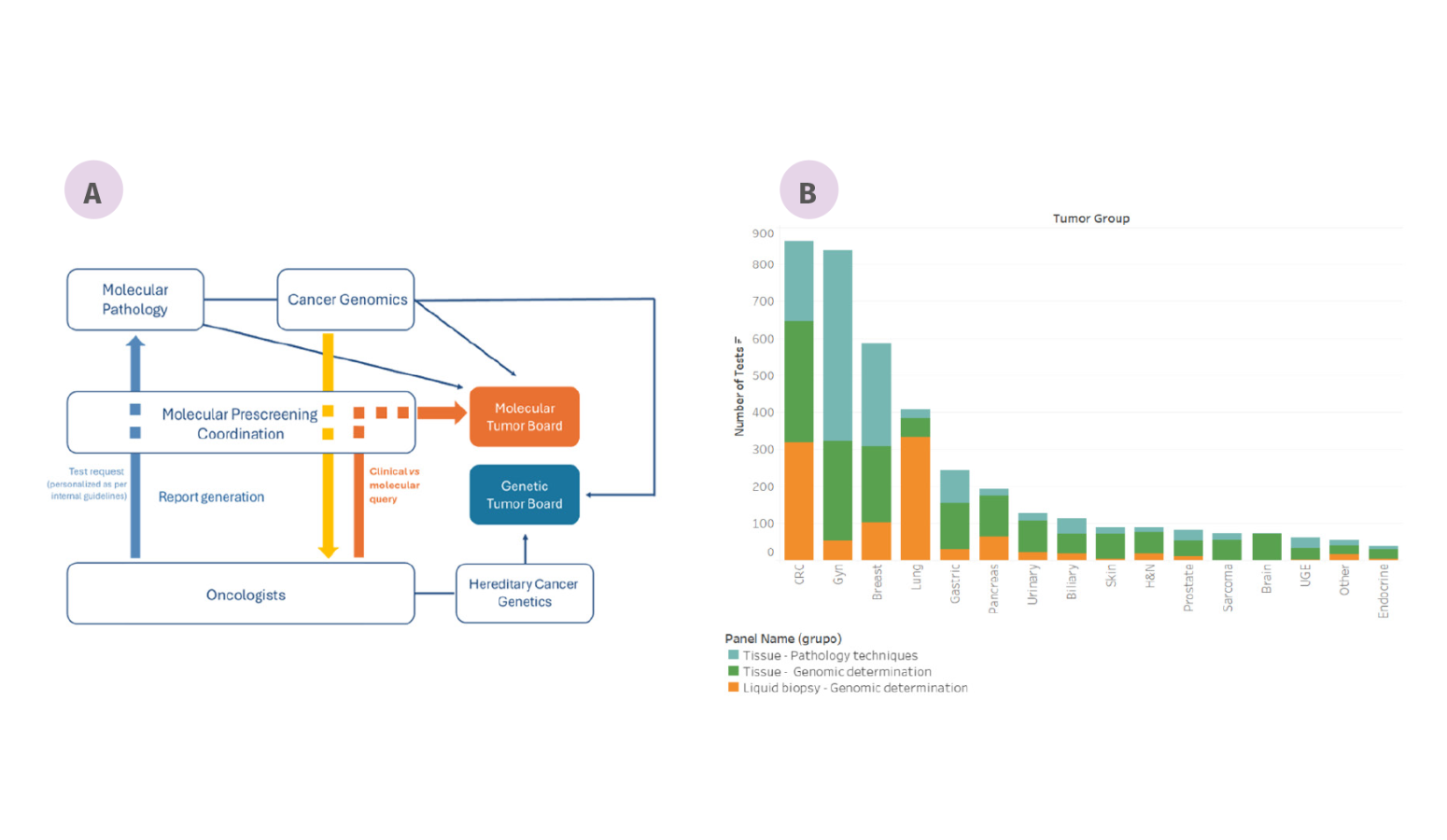
Figure: Molecular Prescreening Program at VHIO. (A) Interrelationship between Genomic and Molecular Pathology laboratories with clinical oncologists, and the functionality of the Prescreening Program. (B) Number of genomic and proteomic tests per tumor type.
- Clinical implementation of advanced molecular diagnostics to optimize the selection of therapies for patients being considered for enrolment in clinical trials.
- Continued medical education with standardized reports of genomic alterations and weekly Molecular Tumor Boards.
- Continued revision and updating of molecular diagnostic tests to include emerging biomarkers for precision oncology.
- Hernando-Calvo A, Vila-Casadesús M, Bareche Y, Gonzalez-Medina A, Abbas-Aghababazadeh F, Lo Giacco D, Martin A, Saavedra O, Brana I, Vieito M, Fasani R, Stagg J, Mancuso F, Haibe-Kains B, Han M, Berche R, Pugh TJ, Mirallas O, Jimenez J, Gonzalez NS, Valverde C, Muñoz-Couselo E, Suarez C, Diez M, Élez E, Capdevila J, Oaknin A, Saura C, Macarulla T, Galceran JC, Felip E, Dienstmann R, Bedard PL, Nuciforo P, Seoane J, Tabernero J, Garralda E, Vivancos A. A pan-cancer clinical platform to predict immunotherapy outcomes and prioritize immuno-oncology combinations in early-phase trials. Med. 2023 Oct 13;4(10):710-727.e5.
- Hernando-Calvo A, Mirallas O, Marmolejo D, Saavedra O, Vieito M, Assaf Pastrana JD, Aguilar S, Bescós C, Lorente J, Giralt J, Benavente S, Temprana-Salvador J, Alberola M, Dienstmann R, Garralda E, Felip E, Villacampa G, Brana I. Nutritional status associates with immunotherapy clinical outcomes in recurrent or metastatic head and neck squamous cell carcinoma patients. Oral Oncol. 2023 May;140:106364.
- Mulet Margalef N, Castillo C, Mosteiro M, Pérez X, Aguilar S, Ruíz-Pace F, Gil M, Cuadra C, Ruffinelli JC, Martínez M, Losa F, Soler G, Teulé À, Castany R, Gallego R, Ruíz A, Garralda E, Élez E, Vivancos A, Tabernero J, Salazar R, Dienstmann R, Santos Vivas C. Genomically matched therapy in refractory colorectal cancer according to ESMO Scale for Clinical Actionability of Molecular Targets: experience of a comprehensive cancer centre network. Mol Oncol. 2023 Sep;17(9):1908-1916.











