VHIO views quality as a broad concept that extends to all areas of the organization’s activity, which goes beyond its purely formal aspects and is developed under the criteria of pragmatism, efficiency and flexibility. VHIO’s Quality Policy is an instrument that reinforces cohesion and corporate identity, and provides a framework of reference for us to carry out our activity in a responsible and sustainable manner.
Quality is key to ensuring competitiveness. VHIO’s Management takes on the objective of achieving optimal levels of Quality linked to the services of biomedical research projects in Oncology, leading to the meeting of customer needs, whether they be internal or external to Vall d’Hebron. It also has the strategic objective of demonstrating the administrative capacity and technical competence of VHIO laboratories for performing clinical analyses linked to the biomedical research that is carried out. Quality, and the entire strategy around it, involves all VHIO staff members and is supported by Management, ensuring the allocation of resources that allow its development and implementation through the appropriate infrastructure, means and the necessary, competent and qualified staff.
VHIO is committed to continuous improvement in its processes and activities, establishing specific improvement objectives and the means to measure our progress. The conclusions of the improvement activities may be reflected in changes to both the Internal Quality Assurance System (IQAS) and the management system, and – if appropriate – to the Quality Policy itself.
Recognition through certification/accreditation
ISO 9001:2015 certification of the quality system for the Management of biomedical research projects in oncology.
ISO 15189:2022 accreditation for the activities defined in the TECHNICAL ANNEX nº1052/LE2022 of the laboratories of:
- Cancer genomics
- Molecular Oncology
- Historical milestones
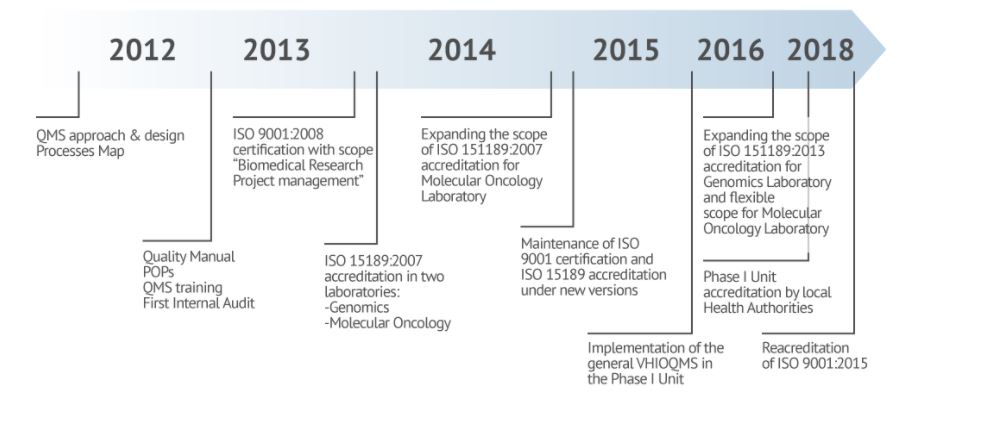
- QMS approach
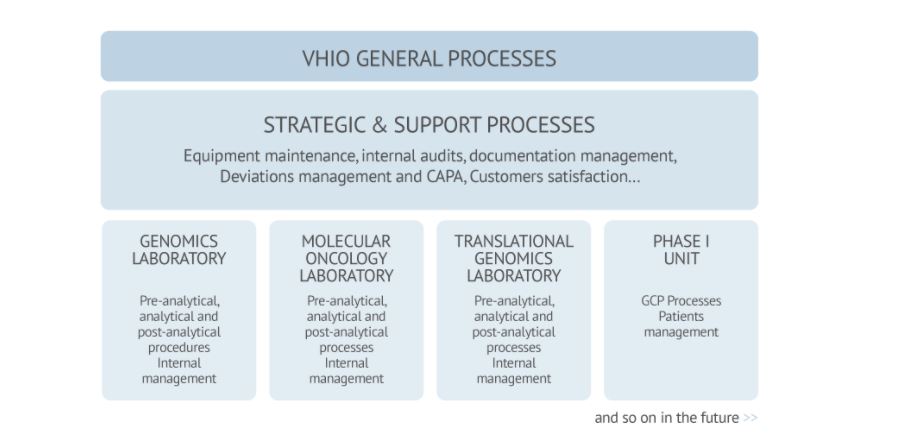
Recognition through Accreditation
Audit of Clinical Trials Units, Generalitat de Catalunya
ENETs Cancer of Excellence
Severo Ochoa Excellence Program
CERCA: A rating
AECC Excellence Program
OECI ACCREDITATION AS AN COMPREHENSIVE CANCER CENTRE
Regardless of the supplier selection that a principal Investigator may make in the context of a project for which he/she is responsible, and which is explained in previous sections of this procedure, VHIO establishes a supplier evaluation system on an annual basis.
The criteria adopted by VHIO for the acceptance of a supplier are:
- Degree of compliance with delivery deadlines.
- Acceptance of VHIO’s conditions for receiving orders.
- Adaptation of the supplier’s product catalogue to the VHIO standard.
- Having made at least one purchase from the supplier in a period not exceeding one year. If this requirement is not met, the IQAS system removes the supplier from the database.
- Not having detected any quality deficiencies in the products/services purchased from the supplier, nor any applicable legal non-compliance.
- Initial review of satisfactory products/services at the time of delivery.
With regard to the quality criteria required of suppliers, these can be:
- Quality of the products: degree of adequacy of the characteristics and technical specifications to the requirements provided by VHIO.
- Warranty periods.
- User training, if appropriate.
- Existence and level of after-sales service.
- Existence and level of customer service.
- Legal/regulatory quality compliance: possessing applicable accreditations, certifications, CE marking, etc.
When selecting a supplier, the most important point is the quality of the items ordered. For this purpose, if necessary, the supplier’s proposal will be subjected to a detailed comparative study of the technical characteristics, sample analysis, etc.
This will be the priority criterion when what is most important is a minimum level of quality required, from the researcher’s point of view (compliance with certain technical characteristics).
In the event that more than one supplier is available that meets the quality requirements, the most economical option will be chosen, provided that the researcher has no explicit preference for one of them as best suiting his/her needs.
In certain circumstances, the selection of a supplier may be based on quality criteria not closely related to the product, such as, for example, after-sales service, warranty periods, image of the product/supplier in the market, existence of customer services, etc.
Whenever possible, we try to ensure that orders for the same product are spread across more than one supplier. The reasons for this are:
- Firstly, to accommodate the preferences of different researchers.
- Secondly, to guarantee the supply in the event of failure by any of the suppliers, guaranteeing that the VHIO is not left without the supply, with the consequent economic and scientific damage that this could cause in the development of the research. The disadvantage of this strategy is that, by dividing the quantity needed among several suppliers, the wholesale discount is lower.
When preparing the Annual Quality Report, the VHIO quality manager collects information from the IQAS system on suppliers and the Excel of non-conformities in order to analyze the suitability of VHIO suppliers.
VHIO’s Quality Department is made up of the following team:
- Sergi Cuadrado – Deputy Manager and Head of the Quality Program
- Javier Fonts – Quality Assurance (Clinical Trials)
- José Jimenez – Molecular Oncology Laboratory Quality Manager
- Deborah Grazia lo Giacco – Cancer Genomics Laboratory Quality Manager
- Miriam Artigas – Quality Assurance (Clinical Trials)
- Gemma Sala – Clinical Trials Quality and Processes Unit Director
















