VHIO’s Cancer Genomics Group serves as a Core Facility. Our laboratory provides access to a range of cutting-edge applications and technologies in cancer genomics including three NextGen Sequencers, MiSeq, NextSeq, and NovaSeq6000 from Illumina, and a ddPCR from BIORAD.
We also pioneer the development of new, fully validated genomic tests that are currently used in the clinical research setting.
We have developed and routinely implemented several NGS-based tests: VHIO300, a 435-gene capture panel (Illumina); Epsilon Panel, an RNA- based enrichment panel for the detection of gene fusions and expression patterns in tumors, and VHIO360, a cutting-edge test for liquid biopsy; technology transfer of the Guardant 360® DX test (Guardant Health). Notably, VHIO was the first cancer research center in Europe to incorporate this avant-garde platform. Aimed at overcoming the limitations associated with traditional tissue biopsy, this technology provides complete genomic results in all solid tumors from a simple blood draw in seven days.
Molecular Prescreening at VHIO is co-led by Ana Vivancos, Head of our group, alongside Paolo Nuciforo, Elena Garralda, and Rodrigo Dienstmann, who lead our Molecular Oncology, Early Clinical Drug Development, and Oncology Data Science – OdysSey Groups, respectively. Through our institutional Advanced Molecular Diagnostics Program – DIAMAV, supported by the FERO Foundation, we perform molecular profiling in patients as potential candidates for enrolment in our phase I clinical trials led by VHIO’s Research Unit for Molecular Therapy of Cancer (UITM) – CaixaResearch, directed by Elena Garralda.
In 2024, we performed tumor molecular profiling of more than 2,300 patients’ tumors. Over 50% of these tests were performed using liquid biopsy. This represents a 25% increase in the use of VHIO360 liquid biopsy testing, reflecting the validity of this tool and the increasing usage of liquid biopsy in the clinical setting. At the end of this year, the Catalan Health Service (CatSalut) selected the VHIO360 liquid biopsy test for comprehensive genomic profiling in baseline metastatic lung cancer patients where classical tumor tissue biopsies are not feasible.
Reflective of our dedication to excellence and quality in the services that we provide, our VHIO300 and VHIO360 panels have received ISO 15189 flexible accreditation, and we aim to obtain the same accreditation for the VHIO Epsilon ɛ00 new custom RNA sequencing panel.
Our research activities focus on the development of novel multiplexed tests that are optimized to FFPE-derived nucleic acids and liquid biopsy. Once developed, these assays are validated and used in both clinical and translational research. We are also involved in several translational research projects. These include the identification of mechanisms of resistance to targeted therapies as well as predictive biomarkers of response to immunotherapy. Based on genome-wide RNA seq and array-based Nanostring technologies, we have developed the VIGex predictive pan cancer platform to help guide patient selection for personalized immune-based therapies.
- Develop and implement large-scale pan-cancer panels to optimize strategies for routine patient prescreening.
- Pioneer the development of cutting-edge tests for comprehensive liquid biopsy analysis.
- Provide cutting-edge applications in cancer genomics by implementing novel technologies and developing new protocols.
- Prioritize translational research projects and promote team science to further strengthen VHIO’s renowned excellence in oncology.
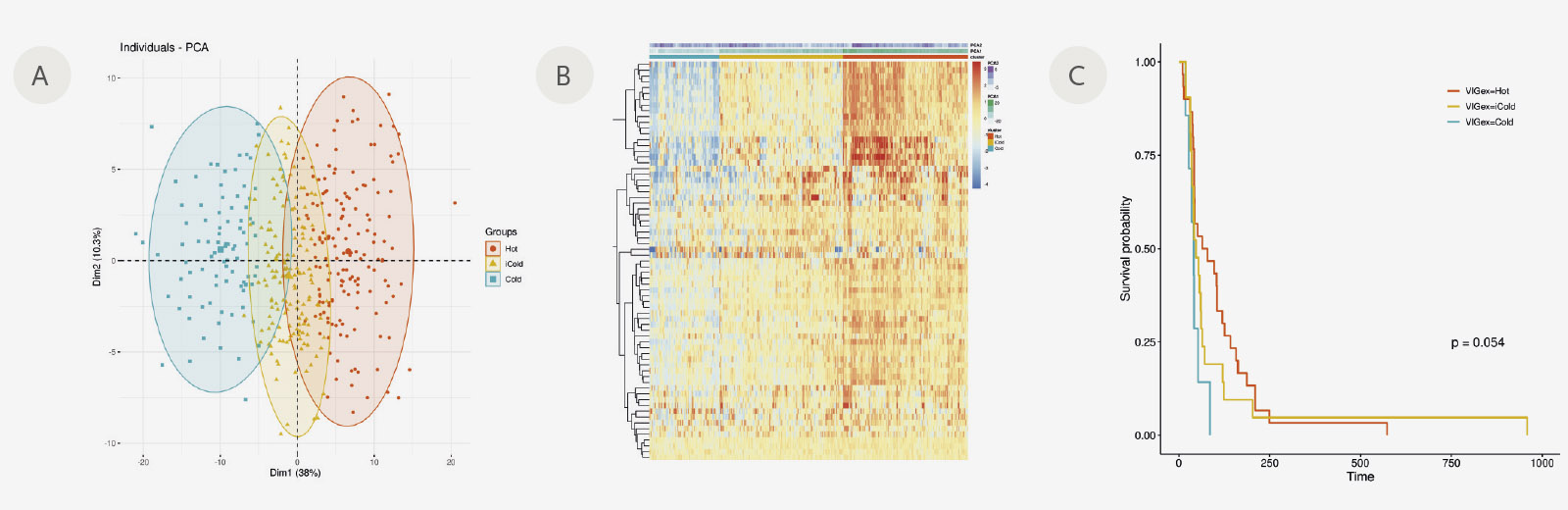
Figure: VIGex classification of 398 cancer metastatic samples according to nCounter (Nanostring) gene expression (69 immuno-related genes). Gene expression values were normalized to the geometric mean expression of 19 housekeeping genes, then log2-transformed and centred around mean. A) PCA showing the 3 clusters identified with PAM (partitioning around medoids) method (Hot, iCold and Cold). B) Heatmap showing relative gene expression and PCA values of the 69 immuno-related genes within Hot, iCold and Cold groups. C) Kaplan-Meier plot showing time to progression of the Hot, iCold and Cold groups of an independent cohort of 58 samples.

Group Leader
Ana Vivancos
Laboratory manager
Agatha Martín
Project manager
Ester Castillo
Postdoctoral Fellow
Eva Rodríguez
Specialized Technicians
Vanessa Bach,
Giuseppe Buono
Ana Onieva
Montserrat Ricart
Ana Vilar
Predoctoral Fellows
Javier Forte
Alberto Hernando
Paula Romero
Bioinformaticians
Elena Campoy
Francesca Catalogne
Marina Gómez
Maria Romero
Núria García
Research Support Technician
Inmaculada Martos
Most relevant scientific publications
- A 14-gene B-cell immune signature in early-stage triple-negative breast cancer (TNBC): a pooled analysis of seven studies. Conte B, Brasó-Maristany F, Hernández AR, Pascual T, Villacampa G, Schettini F, Vidal Losada MJ, Seguí E, Angelats L, Garcia-Fructuoso I, Gómez-Bravo R, Lorman-Carbó N, Paré L, Marín-Aguilera M, Martínez-Sáez O, Adamo B, Sanfeliu E, Fratini B, Falato C, Chic N, Vivancos A, Villagrasa P, Staaf J, Parker JS, Perou CM, Prat A. EBioMedicine. 2024 Apr;102:105043. doi: 10.1016/j.ebiom.2024.105043.
- Five latent factors underlie response to immunotherapy. Usset J, Rosendahl Huber A, Andrianova MA, Batlle E, Carles J, Cuppen E, Elez E, Felip E, Gómez-Rey M, Lo Giacco D, Martinez-Jimenez F, Muñoz-Couselo E, Siu LL, Tabernero J, Vivancos A, Muiños F, Gonzalez-Perez A, Lopez-Bigas N. Nat Genet. 2024 Oct;56(10):2112-2120. doi: 10.1038/s41588-024-01899-0.
- A Phase II, Open-Label, Randomized Trial of Durvalumab With Olaparib or Cediranib in Patients With Mismatch Repair-Proficient Colorectal or Pancreatic Cancer. Hernando-Calvo A, Han M, Ayodele O, Wang BX, Bruce JP, Abbas-Aghababazadeh F, Vila-Casadesús M, Sanz-Garcia E, Yang SYC, Berman HK, Vivancos A, Lam B, Lungu I, Salawu A, Stayner LA, Haibe-Kains B, Bedard PL, Avery L, Razak ARA, Pugh TJ, Spreafico A, Siu LL, Hansen AR. Clin Colorectal Cancer. 2024 Sep;23(3):272-284.e9. doi: 10.1016/j.clcc.2024.05.002.
- Patritumab deruxtecan in HER2-negative breast cancer: part B results of the window-of-opportunity SOLTI-1805 TOT-HER3 trial and biological determinants of early response. Brasó-Maristany F, Ferrero-Cafiero JM, Falato C, Martínez-Sáez O, Cejalvo JM, Margelí M, Tolosa P, Salvador-Bofill FJ, Cruz J, González-Farré B, Sanfeliu E, Òdena A, Serra V, Pardo F, Luna Barrera AM, Arumi M, Guerra JA, Villacampa G, Sánchez-Bayona R, Ciruelos E, Espinosa-Bravo M, Izarzugaza Y, Galván P, Matito J, Pernas S, Vidal M, Santhanagopal A, Sellami D, Esker S, Fan PD, Suto F, Vivancos A, Pascual T, Prat A, Oliveira M. Nat Commun. 2024 Jul 11;15(1):5826. doi: 10.1038/s41467-024-50056-y.
- Prognostic value of HER2DX in early-stage HER2-positive breast cancer: a comprehensive analysis of 757 patients in the Sweden Cancerome Analysis Network-Breast dataset (SCAN-B). Villacampa G, Pascual T, Brasó-Maristany F, Paré L, Martínez-Sáez O, Cortés J, Ciruelos E, Martin M, Conte P, Carey LA, Fernandez A, Harbeck N, Marín-Aguilera M, Vivancos A, Curigliano G, Villagrasa P, Parker JS, Perou CM, Prat A, Tolaney SM. ESMO Open. 2024 Mar;9(3):102388. doi: 10.1016/j.esmoop.2024.102388.
- Use of ctDNA in early breast cancer: analytical validity and clinical potential. Panet F, Papakonstantinou A, Borrell M, Vivancos J, Vivancos A, Oliveira M. NPJ Breast Cancer. 2024 Jun 19;10(1):50. doi: 10.1038/s41523-024-00653-3.
- Analytical validation of HER2DX genomic test for early-stage HER2-positive breast cancer. Marín-Aguilera M, Jares P, Sanfeliu E, Villacampa G, Hernández-Lllán E, Martínez-Puchol AI, Shankar S, González-Farré B, Waks AG, Brasó-Maristany F, Pardo F, Manning DK, Abery JA, Curaba J, Moon L, Gordon O, Galván P, Wachirakantapong P, Castillo O, Nee CM, Blasco P, Senevirathne TH, Sirenko V, Martínez-Sáez O, Aguirre A, Krop IE, Li Z, Spellman P, Metzger Filho O, Polyak K, Michaels P, Puig-Butillé JA, Vivancos A, Matito J, Buckingham W, Perou CM, Villagrasa-González P, Prat A, Parker JS, Paré L. ESMO Open. 2024 Mar;9(3):102903. doi: 10.1016/j.esmoop.2024.102903.
- Clinical Value of Liquid Biopsy in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma During Targeted Therapy. González-Medina A, Vila-Casadesús M, Gomez-Rey M, Fabregat-Franco C, Sierra A, Tian TV, Castet F, Castillo G, Matito J, Martinez P, Miquel JM, Nuciforo P, Pérez-López R, Macarulla T, Vivancos A. Clin Cancer Res. 2024 Oct 1;30(19):4491-4504. doi: 10.1158/1078-0432.CCR-23-3780.
- Combined Transcriptome and Circulating Tumor DNA Longitudinal Biomarker Analysis Associates With Clinical Outcomes in Advanced Solid Tumors Treated With Pembrolizumab. Hernando-Calvo A, Yang SYC, Vila-Casadesús M, Han M, Liu ZA, Berman AHK, Spreafico A, Razak AA, Lheureux S, Hansen AR, Lo Giacco D, Abbas-Aghababazadeh F, Matito J, Haibe-Kains B, Pugh TJ, Bratman SV, Aleshin A, Berche R, Saavedra O, Garralda E, Elston S, Siu LL, Ohashi PS, Vivancos A, Bedard PL. JCO Precis Oncol. 2024 Aug;8:e2400100. doi: 10.1200/PO.24.00100.
- Post-surgery sequelae unrelated to disease progression and chemotherapy revealed in follow-up of patients with stage III colon cancer. Mirandola A, Kudriavtsev A, Cofre Muñoz CI, Navarro RC, Macagno M, Daoud S, Sanchez C, Pastor B, Pisareva E, Marin MS, Ruiz JG, Piris A, Rodriguez AG, Gonzalez NS, Vivancos A, Quarà V, Mellano A, Borghi F, Corti G, Marchiò C, Sapino A, Bartolini A, Crisafulli G, Bardelli A, Di Maio M, Lossaint G, Frayssinoux F, Crapez E, Ychou M, Soler RS, Fenocchio E, Fernandez Calotti PX, Mazard T, Vivas CS, Elez E, Di Nicolantonio F, Thierry AR. EBioMedicine. 2024 Oct;108:105352. doi: 10.1016/j.ebiom.2024.105352.
- Corrigendum to “Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments”: [Annals of Oncology 34 (2023) 543-552]. Ros J, Matito J, Villacampa G, Comas R, Garcia A, Martini G, Baraibar I, Saoudi N, Salvà F, Martin Á, Antista M, Toledo R, Martinelli E, Pietrantonio F, Boccaccino A, Cremolini C, Dienstmann R, Tabernero J, Vivancos A, Elez E. Ann Oncol. 2024 Oct;35(10):922. doi: 10.1016/j.annonc.2023.07.010.
- Circulating tumor DNA, and clinical features to guide rechallenge with BRAF inhibitors in BRAF-V600E mutated metastatic colorectal cancer. Ros J, Vivancos A, Tabernero J, Élez E. Ann Oncol. 2024 Feb;35(2):240-241. doi: 10.1016/j.annonc.2023.10.120.
- Hernando-Calvo A, Vila-Casadesús M, Bareche Y, Gonzalez-Medina A, Abbas-Aghababazadeh F, Lo Giacco D, Martin A, Saavedra O, Brana I, Vieito M, Fasani R, Stagg J, Mancuso F, Haibe-Kains B, Han M, Berche R, Pugh TJ, Mirallas O, Jimenez J, Gonzalez NS, Valverde C, Muñoz-Couselo E, Suarez C, Diez M, Élez E, Capdevila J, Oaknin A, Saura C, Macarulla T, Galceran JC, Felip E, Dienstmann R, Bedard PL, Nuciforo P, Seoane J, Tabernero J, Garralda E, Vivancos A. A pan-cancer clinical platform to predict immunotherapy outcomes and prioritize immuno-oncology combinations in early-phase trials. Med. 2023 Oct 13;4(10):710-727.e5.
- Saura C, Ortiz C, Matito J, Arenas EJ, Suñol A, Martín Á, Córdoba O, Martínez-Sabadell A, García-Ruiz I, Miranda I, Morales-Comas C, Carrasco E, Viaplana C, Peg V, Nuciforo P, Bayó-Puxan N, Gonzalez-Medina A, Miquel JM, Gómez-Rey M, Villacampa G, Arévalo S, Espinosa-Bravo M, Balmaña J, Dienstmann R, Arribas J, Tabernero J, Vivancos A, Sansó M. Early-Stage Breast Cancer Detection in Breast Milk. Cancer Discov. 2023 Oct 5;13(10):2180-2191.
- Prat A, Brasó-Maristany F, Martínez-Sáez O, Sanfeliu E, Xia Y, Bellet M, Galván P, Martínez D, Pascual T, Marín-Aguilera M, Rodríguez A, Chic N, Adamo B, Paré L, Vidal M, Margelí M, Ballana E, Gómez-Rey M, Oliveira M, Felip E, Matito J, Sánchez-Bayona R, Suñol A, Saura C, Ciruelos E, Tolosa P, Muñoz M, González-Farré B, Villagrasa P, Parker JS, Perou CM, Vivancos A. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023 Mar 1;14(1):1157.
- Vivancos A, Tabernero J. Circulating tumor DNA as a novel prognostic indicator. Nat Med. 2022 Nov;28(11):2255-2256.
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118
- Vivancos A, Tabernero J. Circulating tumor DNA as a novel prognostic indicator. Nat Med. 2022 Nov;28(11):2255-2256.
- Boix O, Martinez M, Vidal S, Giménez-Alejandre M, Palenzuela L, Lorenzo-Sanz L, Quevedo L, Moscoso O, Ruiz-Orera J, Ximénez-Embún P, Ciriaco N, Nuciforo P, Stephan-Otto Attolini C, Albà MM, Muñoz J, Tian TV, Varela I, Vivancos A, Ramón Y Cajal S, Muñoz P, Rivas C, Abad M. pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation. Nat Commun. 2022 Nov 11;13(1):6840.
- Garcia-Casado Z, Oaknin A, Mendiola M, Alkorta-Aranburu G, Antunez-Lopez JR, Moreno-Bueno G, Palacios J, Yubero A, Marquez R, Gallego A, Sanchez-Heras AB, Lopez-Guerrero JA, Perez-Segura C, Barretina-Ginesta P, Alarcon J, Gaba L, Marquez A, Matito J, Cueva J, Palacio I, Iglesias M, Arcusa A, Sanchez-Lorenzo L, Guerra-Alia E, Romero I, Vivancos A. Laboratory Cross-Comparison and Ring Test Trial for Tumor BRCA Testing in a Multicenter Epithelial Ovarian Cancer Series: The BORNEO GEICO 60-0 Study. J Pers Med. 2022 Nov 4;12(11):1842.
- Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratú G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez CV, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.2019 Nov 1;30(11):1843.
- Vivancos A, Élez E, Salazar R.Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemoradiotherapy before surgery in patients with locally advanced rectal cancer: is it ready for primetime? Ann Oncol.29: 532-534.
- Capdevila J,Mayor R; Mancuso FF, Iglesias C; Caratù G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez C, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J.Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.29: 1454-1460.
- Cedrés S, Felip E, Cruz C, Martinez de Castro A, Pardo N, Navarro A, Martinez-Marti A, Remon J, Zeron-Medina J, Balmaña J, Llop-Guevara A, Miquel JM, Sansano I, Nuciforo P,Mancuso F, Serra V, Vivancos A. Activity of HSP90 Inhibiton in a Metastatic Lung Cancer Patient With a Germline BRCA1 Mutation. J Natl Cancer Inst.110: 914-917.
- Puig I, Tenbaum SP, Chicote I, Arqués O,Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto A, Aguilar S, Eguizabal C, Caratù G, Prat A, Argilés G, Landolfi S, Casanovas O, Serra V, Villanueva A, Arroyo AG, Terracciano L, Nuciforo P,Seoane J,Recio JA, Vivancos A,Dienstmann R,Tabernero J,Palmer HG.TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest.128: 3887-3905.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S,Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, Lai Z, Polanska UM, Jones GN; Kristel P, de Bustos L, Guzman M,Rodriguez O, Grueso J, Montalban G, Caratú G,Mancuso F, Fasani R, Jiménez J, Howat WJ, Dougherty B, Vivancos A, Nuciforo P, Serres-Créixams X, Rubio IT, Oaknin A, Cadogan E, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Arribas J, Jonkers J, Díez O; O’Connor MJ, Balmaña J,Serra V. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol.29: 1203-1210. IF: 13,926
- Martínez-Ricarte F, Mayor R, Martínez-Sáez E, Rubio-Pérez C, Pineda E, Cordero E, Cicuéndez M, Poca MA, Lopez-Bigas N, Ramón Y Cajal S, Vieito M, Carles J, Tabernero J,Vivancos A, Gallego S, Graus F, Sahuquillo J, Seoane J. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid.Clin Cancer Res.24: 2812-2819.
All publications
- Vidal L, Pando E, Blanco L, Fabregat-Franco C, Castet F, Sierra A, Macarulla T, Balsells J, Charco R, Vivancos A. Liquid biopsy after resection of pancreatic adenocarcinoma and its relation to oncological outcomes. Systematic review and meta-analysis. Cancer Treat Rev. 2023 Nov;120:102604. doi: 10.1016/j.ctrv.2023.102604. Epub 2023 Aug 6. PMID: 37572593.
- Ros J, Vivancos A, Tabernero J, Élez E. Circulating tumor DNA, and clinical features to guide rechallenge with BRAF inhibitors in BRAF-V600E mutated metastatic colorectal cancer. Ann Oncol. 2024 Feb;35(2):240-241. doi: 10.1016/j.annonc.2023.10.120. Epub 2023 Oct 20. PMID: 37866812.
- Saura C, Ortiz C, Matito J, Arenas EJ, Suñol A, Martín Á, Córdoba O, Martínez-Sabadell A, García-Ruiz I, Miranda I, Morales-Comas C, Carrasco E, Viaplana C, Peg V, Nuciforo P, Bayó-Puxan N, Gonzalez-Medina A, Miquel JM, Gómez-Rey M, Villacampa G, Arévalo S, Espinosa-Bravo M, Balmaña J, Dienstmann R, Arribas J, Tabernero J, Vivancos A, Sansó M. Early-Stage Breast Cancer Detection in Breast Milk. Cancer Discov. 2023 Oct 5;13(10):2180-2191.
- Castet F, Fabregat-Franco C, Castillo G, Navarro V, Sierra A, Acosta DA, López-Valbuena D, Dienstmann R, Tabernero J, Vivancos A, Tian TV, Macarulla T. Clinical and genomic characterisation of early-onset pancreatic cancer. Eur J Cancer. 2023 Nov;194:113338. doi: 10.1016/j.ejca.2023.113338. Epub 2023 Sep 9. PMID: 37793216.
- Mulet Margalef N, Castillo C, Mosteiro M, Pérez X, Aguilar S, Ruíz-Pace F, Gil M, Cuadra C, Ruffinelli JC, Martínez M, Losa F, Soler G, Teulé À, Castany R, Gallego R, Ruíz A, Garralda E, Élez E, Vivancos A, Tabernero J, Salazar R, Dienstmann R, Santos Vivas C. Genomically matched therapy in refractory colorectal cancer according to ESMO Scale for Clinical Actionability of Molecular Targets: experience of a comprehensive cancer centre network. Mol Oncol. 2023 Sep;17(9):1908-1916. doi: 10.1002/1878-0261.13444. Epub 2023 Jun 12. PMID: 37097008; PMCID: PMC10483603.
- Cedres S, Serna G, Gonzalez-Medina A, Valdivia A, Assaf-Pastrana JD, Iranzo P, Callejo A, Pardo N, Navarro A, Martinez-Marti A, Priano I, Fasani R, Guardia X, Gonzalo J, Carbonell C, Frigola J, Amat R, Navarro V, Dienstmann R, Vivancos A, Nuciforo P, Felip E. Expression of TILs and Patterns of Gene Expression from Paired Samples of Malignant Pleural Mesothelioma (MPM) Patients. Cancers (Basel). 2023 Jul 14;15(14):3611. doi: 10.3390/cancers15143611. PMID: 37509274; PMCID: PMC10377125.
- Baraibar I, García A, Salvà F, Ros J, Saoudi N, Comas R, Castillo G, Sanchis M, García-Álvarez A, Hernando J, Capdevila J, Castells MR, Martí M, Landolfi S, Espín E, Navalpotro B, Guevara J, Dopazo C, Nuciforo P, Vivancos A, Tabernero J, Élez E. Impact of the COVID-19 pandemic in the early-onset colorectal cancer. Transl Oncol. 2023 Jun;32:101668. doi: 10.1016/j.tranon.2023.101668. Epub 2023 Apr 5. PMID: 37031602; PMCID: PMC10073589.
- Bueno-Muiño C, Echavarría I, López-Tarruella S, Roche-Molina M, Del Monte-Millán M, Massarrah T, Jerez Y, Ayala de la Peña F, García-Sáenz JÁ, Moreno F, Rodríguez-Lescure Á, Malón-Giménez D, Ballesteros García AI, Marín-Aguilera M, Galván P, Brasó-Maristany F, Waks AG, Tolaney SM, Mittendorf EA, Vivancos A, Villagrasa P, Parker JS, Perou CM, Paré L, Villacampa G, Prat A, Martín M. Assessment of a Genomic Assay in Patients With ERBB2-Positive Breast Cancer Following Neoadjuvant Trastuzumab-Based Chemotherapy With or Without Pertuzumab. JAMA Oncol. 2023 Jun 1;9(6):841-846. doi: 10.1001/jamaoncol.2023.0187. PMID: 37103916; PMCID: PMC10141274.
- Waks AG, Ogayo ER, Paré L, Marín-Aguilera M, Brasó-Maristany F, Galván P, Castillo O, Martínez-Sáez O, Vivancos A, Villagrasa P, Villacampa G, Tarantino P, Desai N, Guerriero J, Metzger O, Tung NM, Krop IE, Parker JS, Perou CM, Prat A, Winer EP, Tolaney SM, Mittendorf EA. Assessment of the HER2DX Assay in Patients With ERBB2-Positive Breast Cancer Treated With Neoadjuvant Paclitaxel, Trastuzumab, and Pertuzumab. JAMA Oncol. 2023 Jun 1;9(6):835-840. doi: 10.1001/jamaoncol.2023.0181. PMID: 37103927; PMCID: PMC10141272.
- Carbonell C, Frigola J, Pardo N, Callejo A, Iranzo P, Valdivia A, Priano I, Cedrés S, Martinez-Marti A, Navarro A, Lenza L, Soleda M, Gonzalo-Ruiz J, Vivancos A, Sansó M, Carcereny E, Morán T, Amat R, Felip E. Dynamic changes in circulating tumor DNA assessed by shallow whole-genome sequencing associate with clinical efficacy of checkpoint inhibitors in NSCLC. Mol Oncol. 2023 May;17(5):779-791. doi: 10.1002/1878-0261.13409. Epub 2023 Mar 21. PMID: 36852704; PMCID: PMC10158763.
- Ros J, Matito J, Villacampa G, Comas R, Garcia A, Martini G, Baraibar I, Saoudi N, Salvà F, Martin Á, Antista M, Toledo R, Martinelli E, Pietrantonio F, Boccaccino A, Cremolini C, Dientsmann R, Tabernero J, Vivancos A, Elez E. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann Oncol. 2023 Jun;34(6):543-552. doi: 10.1016/j.annonc.2023.02.016. Epub 2023 Mar 14. PMID: 36921693.
- Prat A, Brasó-Maristany F, Martínez-Sáez O, Sanfeliu E, Xia Y, Bellet M, Galván P, Martínez D, Pascual T, Marín-Aguilera M, Rodríguez A, Chic N, Adamo B, Paré L, Vidal M, Margelí M, Ballana E, Gómez-Rey M, Oliveira M, Felip E, Matito J, Sánchez-Bayona R, Suñol A, Saura C, Ciruelos E, Tolosa P, Muñoz M, González-Farré B, Villagrasa P, Parker JS, Perou CM, Vivancos A. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023 Mar 1;14(1):1157. doi: 10.1038/s41467-023-36801-9. PMID: 36859416; PMCID: PMC9977734.
- Serra-Camprubí Q, Verdaguer H, Oliveros W, Lupión-Garcia N, Llop-Guevara A, Molina C, Vila-Casadesús M, Turpin A, Neuzillet C, Frigola J, Querol J, Yáñez-Bartolomé M, Castet F, Fabregat-Franco C, Escudero-Iriarte C, Escorihuela M, Arenas EJ, Bernadó-Morales C, Haro N, Giles FJ, Pozo ÓJ, Miquel JM, Nuciforo PG, Vivancos A, Melé M, Serra V, Arribas J, Tabernero J, Peiró S, Macarulla T, Tian TV. Human Metastatic Cholangiocarcinoma Patient-Derived Xenografts and Tumoroids for Preclinical Drug Evaluation. Clin Cancer Res. 2023 Jan 17;29(2):432-445. doi: 10.1158/1078-0432.CCR-22-2551. PMID: 36374558; PMCID: PMC9873249.
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118. doi: 10.3390/ijms24010118. PMID: 36613564; PMCID: PMC9820517.
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118. doi: 10.3390/ijms24010118. PMID: 36613564; PMCID: PMC9820517.
- Palomero J, Panisello C, Lozano-Rabella M, Tirtakasuma R, Díaz-Gómez J, Grases D, Pasamar H, Arregui L, Dorca Duch E, Guerra Fernández E, Vivancos A, de Andrea CE, Melero I, Ponce J, Vidal A, Piulats JM, Matias-Guiu X, Gros A. Biomarkers of tumor-reactive CD4<sup>+</sup> and CD8<sup>+</sup> TILs associate with improved prognosis in endometrial cancer. J Immunother Cancer. 2022 Dec;10(12):e005443. doi: 10.1136/jitc-2022-005443. PMID: 36581331; PMCID: PMC9806064.
- Garcia-Casado Z, Oaknin A, Mendiola M, Alkorta-Aranburu G, Antunez-Lopez JR, Moreno-Bueno G, Palacios J, Yubero A, Marquez R, Gallego A, Sanchez-Heras AB, Lopez-Guerrero JA, Perez-Segura C, Barretina-Ginesta P, Alarcon J, Gaba L, Marquez A, Matito J, Cueva J, Palacio I, Iglesias M, Arcusa A, Sanchez-Lorenzo L, Guerra-Alia E, Romero I, Vivancos A. Laboratory Cross-Comparison and Ring Test Trial for Tumor <i>BRCA</i> Testing in a Multicenter Epithelial Ovarian Cancer Series: The BORNEO GEICO 60-0 Study. J Pers Med. 2022 Nov 4;12(11):1842. doi: 10.3390/jpm12111842. PMID: 36579549; PMCID: PMC9698073.
- Guarneri V, Bras-Maristany F, Dieci MV, Griguolo G, Par L, Mar Ín-Aguilera M, Miglietta F, Bottosso M, Giorgi CA, Blasco P, Castillo O, Galv N P, Vivancos A, Villagrasa P, Parker JS, Perou CM, Conte P, Prat A. HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: A correlative analysis from the PerELISA trial. EBioMedicine. 2022 Nov;85:104320. doi: 10.1016/j.ebiom.2022.104320. Epub 2022 Oct 29. PMID: 36374768; PMCID: PMC9626543.
- Devis-Jauregui L, Vidal A, Plata-Peña L, Santacana M, García-Mulero S, Bonifaci N, Noguera-Delgado E, Ruiz N, Gil M, Dorca E, Llobet FJ, Coll-Iglesias L, Gassner K, Martinez-Iniesta M, Rodriguez-Barrueco R, Barahona M, Marti L, Viñals F, Ponce J, Sanz-Pamplona R, Piulats JM, Vivancos A, Matias-Guiu X, Villanueva A, Llobet-Navas D. Generation and Integrated Analysis of Advanced Patient-Derived Orthoxenograft Models (PDOX) for the Rational Assessment of Targeted Therapies in Endometrial Cancer. Adv Sci (Weinh). 2022 Nov 14;10(1):e2204211. doi: 10.1002/advs.202204211. Epub ahead of print. PMID: 36373729; PMCID: PMC9811454.
- Boix O, Martinez M, Vidal S, Giménez-Alejandre M, Palenzuela L, Lorenzo-Sanz L, Quevedo L, Moscoso O, Ruiz-Orera J, Ximénez-Embún P, Ciriaco N, Nuciforo P, Stephan-Otto Attolini C, Albà MM, Muñoz J, Tian TV, Varela I, Vivancos A, Ramón Y Cajal S, Muñoz P, Rivas C, Abad M. pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation. Nat Commun. 2022 Nov 11;13(1):6840. doi: 10.1038/s41467-022-34529-6. PMID: 36369429; PMCID: PMC9652315.
- Vivancos A, Tabernero J. Circulating tumor DNA as a novel prognostic indicator. Nat Med. 2022 Nov;28(11):2255-2256. doi: 10.1038/s41591-022-02068-8. PMID: 36357679.
- Elez E, Ros J, Fernández J, Villacampa G, Moreno-Cárdenas AB, Arenillas C, Bernatowicz K, Comas R, Li S, Kodack DP, Fasani R, Garcia A, Gonzalo-Ruiz J, Piris-Gimenez A, Nuciforo P, Kerr G, Intini R, Montagna A, Germani MM, Randon G, Vivancos A, Smits R, Graus D, Perez-Lopez R, Cremolini C, Lonardi S, Pietrantonio F, Dienstmann R, Tabernero J, Toledo RA. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat Med. 2022 Oct;28(10):2162-2170. doi: 10.1038/s41591-022-01976-z. Epub 2022 Sep 12. PMID: 36097219; PMCID: PMC9556333.
- Zurita AJ, Graf RP, Villacampa G, Raskina K, Sokol E, Jin D, Antonarakis ES, Li G, Huang RSP, Casanova-Salas I, Vivancos A, Carles J, Ross JS, Schrock AB, Oxnard GR, Mateo J. Genomic Biomarkers and Genome-Wide Loss-of-Heterozygosity Scores in Metastatic Prostate Cancer Following Progression on Androgen-Targeting Therapies. JCO Precis Oncol. 2022 Jul;6:e2200195. doi: 10.1200/PO.22.00195. PMID: 35820087; PMCID: PMC9307307.
- Frigola J, Carbonell C, Irazno P, Pardo N, Callejo A, Cedres S, Martinez-Marti A, Navarro A, Soleda M, Jimenez J, Hernandez-Losa J, Vivancos A, Felip E, Amat R. High levels of chromosomal aberrations negatively associate with benefit to checkpoint inhibition in NSCLC. J Immunother Cancer. 2022 Apr;10(4):e004197. doi: 10.1136/jitc-2021-004197. Erratum in: J Immunother Cancer. 2022 Jun;10(6): PMID: 35477861; PMCID: PMC9047699.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. Author Correction: The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 May;3(5):649. doi: 10.1038/s43018-022-00378-x. Erratum for: Nat Cancer. 2022 Feb;3(2):251-261. PMID: 35449310; PMCID: PMC9135626.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 Feb;3(2):251-261. doi: 10.1038/s43018-022-00332-x. Epub 2022 Feb 24. Erratum in: Nat Cancer. 2022 May;3(5):649. PMID: 35221333; PMCID: PMC8882467.
- Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, Espinosa-Bravo M, Peg V, Vivancos A, Saura C, Villacampa G, Oliveira M. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102362. doi: 10.1016/j.ctrv.2022.102362. Epub 2022 Feb 18. PMID: 35219090.
- Verdaguer H, Saurí T, Acosta DA, Guardiola M, Sierra A, Hernando J, Nuciforo P, Miquel JM, Molero C, Peiró S, Serra-Camprubí Q, Villacampa G, Aguilar S, Vivancos A, Tabernero J, Dienstmann R, Macarulla T. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin Cancer Res. 2022 Apr 14;28(8):1662-1671. doi: 10.1158/1078-0432.CCR-21-2384. PMID: 35042699.
- Fernández Montes A, Élez E, Vivancos A, Martínez N, González P, Covela M, de la Cámara J, Cousillas A, Méndez JC, Graña B, Aranda E. Monitoring of RAS mutant clones in plasma of patients with RAS mutant metastatic colorectal cancer. Clin Transl Oncol. 2022 Jun;24(6):1209-1214. doi: 10.1007/s12094-021-02767-7. Epub 2022 Jan 7. PMID: 34997474; PMCID: PMC9107427.
- Prat A, Guarneri V, Pascual T, Brasó-Maristany F, Sanfeliu E, Paré L, Schettini F, Martínez D, Jares P, Griguolo G, Dieci MV, Cortés J, Llombart- Cussac A, Conte B, Marín-Aguilera M, Chic N, Puig-Butillé JA, Martínez A, Galván P, Tsai YH, González-Farré B, Mira A, Vivancos A, Villagrasa P, Parker JS, Conte P, Perou CM. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine. 2022 Jan;75:103801. doi: 10.1016/j.ebiom.2021.103801. Epub 2022 Jan 3. PMID: 34990895; PMCID: PMC8741424.
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High <i>FGFR1-4</i> mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149. doi: 10.1158/1078-0432.CCR-21-1810. Epub 2021 Sep 30. PMID: 34593528.
- Pernas S, Villagrasa P, Vivancos A, Scaltriti M, Rodón J, Burgués O, Nuciforo P, Canes J, Paré L, Dueñas M, Vidal M, Cejalvo JM, Perelló A, Llommbard-Cussac A, Dorca J, Montaño A, Pascual T, Oliveira M, Ribas G, Rapado I, Prat A, Ciruelos E. First Nationwide Molecular Screening Program in Spain for Patients With Advanced Breast Cancer: Results From the AGATA SOLTI-1301 Study. Front Oncol. 2021 Nov 4;11:744112.
- Brasó-Maristany F, Sansó M, Chic N, Martínez D, González-Farré B, Sanfeliu E, Ghiglione L, Carcelero E, Garcia-Corbacho J, Sánchez M, Soy D, Jares P, Peg V, Saura C, Muñoz M, Prat A, Vivancos A. Case Report: A Case Study Documenting the Activity of Atezolizumab in a PD-L1-Negative Triple-Negative Breast Cancer. Front Oncol. 2021 Sep 20;11:710596.
- Martinez-Marti A, Felip E, Mancuso FM, Caratú G, Matito J, Nuciforo P, Sansano I, Diaz-Mejia N, Cedrés S, Callejo A, Iranzo P, Pardo N, Miquel JM, Navarro A, Vivancos A, Sansó M. Genetic evolution to tyrosine kinase inhibitory therapy in patients with EGFR-mutated non-small-cell lung cancer. Br J Cancer. 2021 Nov;125(11):1561-1569.
- Saura C, Matito J, Oliveira M, Wildiers H, Brufksy AM, Waters SH, Hurvitz SA, Moy B, Kim SB, Gradishar WJ, Queiroz GS, Cronemberger E, Wallweber GJ, Bebchuk J, Keyvanjah K, Lalani AS, Bryce R, Vivancos A, Eli LD, Delaloge S. Biomarker Analysis of the Phase III NALA Study of Neratinib + Capecitabine versus Lapatinib + Capecitabine in Patients with Previously Treated Metastatic Breast Cancer. Clin Cancer Res. 2021 Nov 1;27(21):5818-5827.
- Ogbah Z, Mancuso FM, Vivancos A. MYC Copy Number Detection in Clinical Samples Using a Digital DNA-Hybridization and Detection Method. Methods Mol Biol. 2021;2318:321-336.
- Kagawa Y, Elez E, García-Foncillas J, Bando H, Taniguchi H, Vivancos A, Akagi K, García A, Denda T, Ros J, Nishina T, Baraibar I, Komatsu Y, Ciardiello D, Oki E, Kudo T, Kato T, Yamanaka T, Tabernero J, Yoshino T. Combined Analysis of Concordance between Liquid and Tumor Tissue Biopsies for RAS Mutations in Colorectal Cancer with a Single Metastasis Site: The METABEAM Study. Clin Cancer Res. 2021 May 1;27(9):2515-2522.
- Frigola J, Navarro A, Carbonell C, Callejo A, Iranzo P, Cedrés S, Martinez-Marti A, Pardo N, Saoudi-Gonzalez N, Martinez D, Jimenez J, Sansano I, Mancuso FM, Nuciforo P, Montuenga LM, Sánchez-Cespedes M, Prat A, Vivancos A, Felip E, Amat R. Molecular profiling of long-term responders to immune checkpoint inhibitors in advanced non-small cell lung cancer. Mol Oncol. 2021 Apr;15(4):887-900.
- Targeted multiplex proteomics for molecular prescreening and biomarker discovery in metastatic colorectal cancer. Serna G, Ruiz-Pace F, Cecchi F, Fasani R, Jimenez J, Thyparambil S, Landolfi S, Elez E, Vivancos A, Hembrough T, Tabernero J, Dienstmann R, Nuciforo P. Sci Rep. 2019 Sep 19;9(1):13568.
- Early evolutionary divergence between papillary and anaplastic thyroid cancers. Capdevila J, Mayor R, Mancuso FM, Iglesias C, Caratú G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez CV, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Ann Oncol. 2019 Nov 1;30(11):1843.
- Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer.Elez E, Chianese C, Sanz-García E, Martinelli E, Noguerido A, Mancuso FM, Caratù G, Matito J, Grasselli J, Cardone C, Esposito Abate R, Martini G, Santos C, Macarulla T, Argilés G, Capdevila J, Garcia A, Mulet N, Maiello E, Normanno N, Jones F, Tabernero J, Ciardello F, Salazar R, Vivancos A. Mol Oncol. 2019 Sep;13(9):1827-1835.
- Comparison of the Clinical Sensitivity of the Idylla Platform and the OncoBEAM RAS CRC Assay for KRAS Mutation Detection in Liquid Biopsy Samples. Vivancos A, Aranda E, Benavides M, Élez E, Gómez-España MA, Toledano M, Alvarez M, Parrado MRC, García-Barberán V, Diaz-Rubio E. Sci Rep. 2019 Jun 20;9(1):8976.
- Genomic heterogeneity and efficacy of PI3K pathway inhibitors in patients with gynaecological cancer. Rodriguez-Freixinos V, Ruiz-Pace F, Fariñas-Madrid L, Garrido-Castro AC, Villacampa G, Nuciforo P, Vivancos A, Dienstmann R, Oaknin A. ESMO Open. 2019 Mar 8;4(2):e000444.
- Vivancos A, Élez E, Salazar R. Circulating cell-free DNA as predictor of treatment failure after neoadjuvant chemoradiotherapy before surgery in patients with locally advanced rectal cancer: is it ready for primetime? Ann Oncol.29: 532-534.
- Capdevila J, Mayor R; Mancuso FF, Iglesias C; Caratù G, Matos I, Zafón C, Hernando J, Petit A, Nuciforo P, Cameselle-Teijeiro JM, Álvarez C, Recio JA, Tabernero J, Matias-Guiu X, Vivancos A, Seoane J. Early evolutionary divergence between papillary and anaplastic thyroid cancers. Ann Oncol.29: 1454-1460.
- Cedrés S, Felip E, Cruz C, Martinez de Castro A, Pardo N, Navarro A, Martinez-Marti A, Remon J, Zeron-Medina J, Balmaña J, Llop-Guevara A, Miquel JM, Sansano I, Nuciforo P, Mancuso F, Serra V, Vivancos A. Activity of HSP90 Inhibiton in a Metastatic Lung Cancer Patient With a Germline BRCA1 Mutation. J Natl Cancer Inst. 110: 914-917.
- Puig I, Tenbaum SP, Chicote I, Arqués O, Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto A, Aguilar S, Eguizabal C, Caratù G, Prat A, Argilés G, Landolfi S, Casanovas O, Serra V, Villanueva A, Arroyo AG, Terracciano L, Nuciforo P, Seoane J, Recio JA, Vivancos A, Dienstmann R, Tabernero J, Palmer HG. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest. 128: 3887-3905.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, Lai Z, Polanska UM, Jones GN; Kristel P, de Bustos L, Guzman M, Rodriguez O, Grueso J, Montalban G, Caratú G, Mancuso F, Fasani R, Jiménez J, Howat WJ, Dougherty B, Vivancos A, Nuciforo P, Serres-Créixams X, Rubio IT, Oaknin A, Cadogan E, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Arribas J, Jonkers J, Díez O; O’Connor MJ, Balmaña J, Serra V. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 29: 1203-1210.
- Martínez-Ricarte F, Mayor R, Martínez-Sáez E, Rubio-Pérez C, Pineda E, Cordero E, Cicuéndez M, Poca MA, Lopez-Bigas N, Ramón Y Cajal S, Vieito M, Carles J, Tabernero J, Vivancos A, Gallego S, Graus F, Sahuquillo J, Seoane J. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid. Clin Cancer Res. 24: 2812-2819.
- Martinez-Marti A, Felip E, Matito J, Mereu E, Navarro A, Cedrés S, Pardo N, Martinez de Castro A, Remon J, Miquel J M, Guillaumet-Adkins A, Nadal E, Rodriguez-Esteban G, Arqués O, Fasani R, Nuciforo P, Heyn H, Villanueva A, Palmer H G, Vivancos A. Dual MET and ERBB inhibition overcomes intratumor plasticity in osimertinib-resistant-advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2017 Oct 1;28(10):2451-2457.
- García-Foncillas J, Alba E, Aranda E, Díaz-Rubio E, López-López R, Tabernero J, Vivancos A. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol. 2017 Dec 1;28(12):2943-2949.
- Dienstmann R, Elez E, Argiles G, Matos I, Sanz-Garcia E, Ortiz C, Macarulla T, Capdevila J, Alsina M, Sauri T, Verdaguer H, Vilaro M, Ruiz-Pace F, Viaplana C, Garcia A, Landolfi S, Palmer HG, Nuciforo P, Rodon J, Vivancos A, Tabernero J. Analysis of mutant allele fractions in driver genes in colorectal cancer – biological and clinical insights. Mol Oncol. 2017 Sep;11(9):1263-1272.
- Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, Vidal J, Garcia M, Viéitez JM, Paéz D, Falcó E, Lopez Lopez C, Aranda E, Jones F, Sikri V, Nuciforo P, Fasani R, Tabernero J, Montagut C, Azuara D, Dienstmann R, Salazar R, Vivancos A. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2017 Jun 1;28(6):1294-1301.
- Pérez-Alea M, Vivancos A, Caratú G, Matito J, Ferrer B, Hernandez-Losa J, Cortés J, Muñoz E, Garcia-Patos V, Recio JA. Genetic profile of GNAQ-mutated blue melanocytic neoplasms reveals mutations in genes linked to genomic instability and the PI3K pathway. Oncotarget. 2016 May 10;7(19):28086-95. doi: 10.18632/oncotarget.8578.
- Arqués O, Chicote I, Puig I, Tenbaum SP, Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J, Silberschmidt D, Rodon J, Landolfi S, Prat A, Espín E, Charco R, Nuciforo P, Vivancos A, Shao W, Tabernero J, Palmer HG. Tankyrase Inhibition Blocks Wnt/β-Catenin Pathway and Reverts Resistance to PI3K and AKT Inhibitors in the Treatment of Colorectal Cancer. Clin Cancer Res. 2016 Feb 1;22(3):644-56. doi: 10.1158/1078-0432.CCR-14-3081.
- Ibarrola-Villava M, Fleitas T, Llorca-Cardeñosa MJ, Mongort C, Alonso E, Navarro S, Burgues O, Vivancos A, Cejalvo JM, Perez-Fidalgo JA, Roselló S, Ribas G, Cervantes A. Determination of somatic oncogenic mutations linked to target-based therapies using MassARRAY technology. Oncotarget. 2016 Apr 19;7(16):22543-55. doi: 10.18632/oncotarget.8002.
- Alves-Rodrigues I, Ferreira PG, Moldón A, Vivancos AP, Hidalgo E, Guigó R, Ayté J. Spatiotemporal Control of Forkhead Binding to DNA Regulates the Meiotic Gene Expression Program. Cell Rep. 2016 Feb 2;14(4):885-95. doi: 10.1016/j.celrep.2015.12.074.
- Vivancos A, Caratú G, Matito J, Muñoz E, Ferrer B, Hernández-Losa J, Bodet D, Pérez-Alea M, Cortés J, Garcia-Patos V, Recio JA. Genetic evolution of nevus of Ota reveals clonal heterogeneity acquiring BAP1 and TP53 mutations. Pigment Cell Melanoma Res. 2016 Mar;29(2):247-53. doi: 10.1111/pcmr.12452.
- Yus E, Güell M, Vivancos AP, Chen WH, Lluch-Senar M, Delgado J, Gavin AC, Bork P, Serrano L. Transcription start site associated RNAs in bacteria. Mol. Syst. Biol. 2012; 8: 585
- Esteve-Codina A, Kofler R, Himmelbauer H, Ferretti L, Vivancos AP, Groenen MA, Folch JM, Rodríguez MC, Pérez-Enciso M. Partial short-read sequencing of a highly inbred Iberian pig and genomics inference thereof. Heredity (Edinb) 2011 Sep; 107(3): 256-64
- Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos AP, Ayté J, Hidalgo E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010 Mar; 29(5): 981-91
- Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayté J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc. Natl. Acad. Sci. U.S.A. 2005 Jun; 102(25): 8875-80
- Vivancos AP, Castillo EA, Jones N, Ayté J, Hidalgo E. Activation of the redox sensor Pap1 by hydrogen peroxide requires modulation of the intracellular oxidant concentration. Mol. Microbiol. 2004 Jun; 52(5): 1427-35
- Castillo EA, Vivancos AP, Jones N, Ayté J, Hidalgo E. Schizosaccharomyces pombe cells lacking the Ran-binding protein Hba1 show a multidrug resistance phenotype due to constitutive nuclear accumulation of Pap1. J. Biol. Chem. 2003 Oct; 278(42): 40565-72
- Copy Number Alterations (CNA) as a predictive and prognostic biomarker to determine Homologous Recombination Deficiency (HRD) status. Funded by: Agencia de Gestió d’Ajuts Universitaris i de Recerca (AGAUR). Reference: 2024 LLAV 00126. 12/01/2024 – 05/31/2025. PI: Ana Vivancos
- Avanzando hacia la implementación clínica: Estudio de las bases moleculares de la liberación de DNA tumoral en sangre. FIS-ISCIII. (PI20/01112). PI: Ana Vivancos. 01/01/2021 – 31/12/2023. Proyecto CPP2021 CPP2021 -009037 financiado por MCIN/AEI /10.13039/501100011033 y la Unión Europea NextGenerationEU / PRTR

- CGI-Clinics. Data-Driven Cancer Genome Interpretation for Personalized Cancer Treatment. Funding: HORIZON-HLTH-2021-CARE-05-02. HORIZON-RIA 101057509. Award period: 01/11/2022 – 31/10/2027. Principal Investigator: Ana Vivancos.
- Estudio de las bases moleculares de la liberación de DNA tumoral en sangre. FIS-ISCIII. (PI20/01112). PI: Ana Vivancos. 01/01/2021 – 31/12/2023.
- A pivotal study of derazantinib in patients with inoperable or advanced intrahepatic cholangiocarcinoma and FGFR2 gene fusions or FGFR2 gene mutations or amplifications. PI: Ana Vivancos. 22/02/2021 – 31/12/2021.
- Phase II Study of Avelumab plus chemotherapy in the peri-operative treatment for patients with resectable Gastric cancer (GC) or Gastroesophageal Junction cancer (GECJ). Merck Healthcare KGaA. PI: Ana Vivancos. 10/04/2019 – 31/12/2026.
- CeLac and European consortium for a personalized medicine approach to Gastric Cancer-LEGACy. European Commission-LEGACy. PI: Ana Vivancos. 01/01/2019-31/12/2022.
- Estudio de la microbiota, de Fusobacterium nucleatum y de la correlación con las vías de los receptores de Toll-like en tumores neuroendocrinos de intestino delgado. PI: Ana Vivancos. 21/07/2021 -3 1/12/2022.
- Olaparib and durvalumab (MEDI4736) in patients with metastatic pancreatic cancer and DNA Damage Repair genes alterations. PI: Ana Vivancos. 27/10/2021-31/12/2022.
- Evaluate the ability to detect and concordance level between FGFR2 fusions in tumor samples and cfDNA. Co-Principal Investigator: Ana Vivancos. Funding: Incyte Biosciences International. Award period: 07/10/2020 – 31/12/2022.
- Moving Liquid biopsy beyond current applications: study of prognostic and predictivevalues of circulating tumor DNA in metastatic colorectal cancer. Principal Investigator: Ana Vivancos. Funding: Asociación Española Contra el Cáncer. Reference: PROYE18031VIVA. Award period: 01/11/2018 – 31/10/2022.
- ctDNA in breast milk for early detection of pregnancy associated breast cancer. Principal investigator: Cristina Saura & Ana Vivancos. Funding: Fundación FERO – GHD. Award period: 01/07/2020-20/06/2022.
- CeLac and European consortium for a personalized medicine approach to Gastric Cancer-LEGACy. Principal Investigator: Ana Vivancos. Funding: European Commission. Award period: 01/01/2019 – 31/12/2022.
- Tumores primarios múltiples en pacientes con cáncer de pulmón: elaboración de un perfil molecular integral para elucidar orígenes genéticos comunes. Principal Investigator: Ana Vivancos. Funding: Fundación Científica de la Asociación Española Contra el Cáncer (AECC). Código proyecto: INVES19056SANS. Award period: 01/12/2019 – 30/11/2023.