
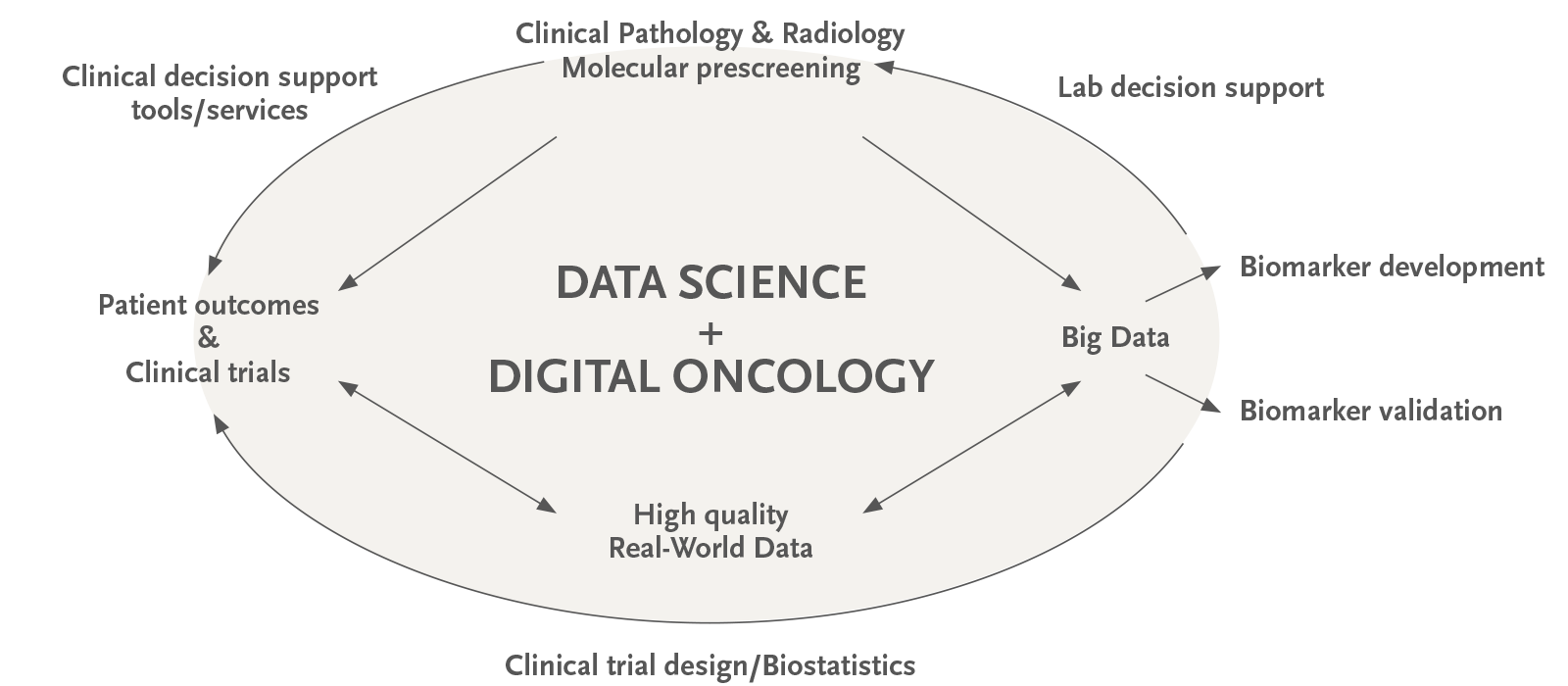
El Grup ODysSey del VHIO promou la recerca translacional en oncologia de precisió mitjançant la integració de dades de perfils moleculars del càncer amb els resultats clínics dels pacients oncològics tractats a l’Hospital Universitari Vall d’Hebron (HUVH).
El nostre grup dissenya i manté bases de dades clinicocomoleculars integrals i desenvolupa sistemes personalitzats de suport a la presa de decisions per als investigadors interessats en les anàlisis correlatives per a la generació d’hipòtesis i la validació de biomarcadors. També donem suport als investigadors en la identificació de pacients escollibles per a estudis translacionals, el càlcul de la mida de la mostra, el disseny de l’assaig clínic, la captura de dades amb formularis electrònics de notificació de casos i les anàlisis estadístiques.
Participem en projectes internacionals d’anàlisi de dades multiòmiques del món real, fomentem la recerca col·laborativa en oncologia computacional i ens dediquem a posar en contacte investigadors oncològics que treballen en l’elaboració de models de predicció i de pronòstic, la identificació d’impulsors del càncer , la determinació del subtipus molecular, l’heterogeneïtat de les metàstasis primàries, les firmes de microambients i la modulabilitat farmacològica en tumors sòlids.
En col·laboració amb el Grup de Genòmica del Càncer del VHIO, el Grup d’Oncologia Molecular i el Grup de Desenvolupament Clínic Precoç de Fàrmacs, codirigim el Programa de PreScreening Molecular del VHIO. A més, despleguem eines informàtiques per explorar i visualitzar dades multiòmiques amb finalitats de recerca i mantenim aplicacions web en el camp de l’atenció oncològica de precisió i l’aparellament d’assajos clínics.
Facilitar els estudis correlatius clinicocomoleculars al VHIO:
- Desenvolupament i manteniment de bases de dades clinicocomoleculars i programes informàtics de suport a la presa de decisions com a recursos per a metges, patòlegs moleculars i investigadors translacionals.
- Oferir orientació a oncòlegs mèdics i biòlegs del càncer sobre el disseny i la interpretació dels estudis correlatius de biomarcadors, així com sobre el desenvolupament i la validació de proves basades en l’òmica que tinguin una aplicació clínica directa.
Promoure la medicina basada en l’evidència i el reclutament per a assaigs clínics:
- Formació mèdica continuada amb informes estandarditzats d’alteracions genòmiques i reunions setmanals del Consell de Tumors Moleculars. Facilitem l’intercanvi de dades entre un gran nombre d’experts per a la revisió dels historials mèdics dels pacients i els perfils moleculars del càncer per informar i orientar amb més precisió les decisions terapèutiques.
- Desenvolupament i manteniment d’OncoTrialsTrack (https://oncotrialstrack.vhio.net), una eina que facilita als professionals sanitaris la difícil i llarga tasca de trobar l’assaig clínic més adequat per als pacients amb càncer i posar-se en contacte amb els investigadors per discutir i remetre el cas.
Recerca col·laborativa sobre macrodades i dades reals:
- Generació de bases de dades d’alta qualitat que permetin estudiar associacions complexes entre l’òmica tumoral, la sensibilitat als fàrmacs i el resultat del pacient.
- Fomentar les interaccions entre científics d’oncologia computacional i investigadors preclínics i clínics per promoure la identificació de subtipus de càncer i factors de farmacibilitat.
Cap de grup
Rodrigo Dientsmann
Coordinadora Projectes
Cristina Viaplana
Analistas de dades
Fiorella Ruiz
Raquel Comas
Gloria Castillo
Laia Joval
Armando Mel
Christina Zatse
Haitham Alatoom
Sandra Martinez
Eduardo García
Oncolog Medic
Pablo Cresta
Kira Raskina
Publicacions científiques més rellevants
- Villacampa G, Cresta Morgado P, Navarro V, Viaplana C, Dienstmann R. Comprehensive evaluation of surrogate endpoints to predict overall survival in trials with PD1/PD-L1 immune checkpoint inhibitors plus chemotherapy. Cancer Treat Rev. 2023 May;116:102542.
- Villacampa G, Tung NM, Pernas S, Paré L, Bueno-Muiño C, Echavarría I, López-Tarruella S, Roche-Molina M, Del Monte-Millán M, Marín-Aguilera M, Brasó-Maristany F, Waks AG, Pascual T, Martínez-Sáez O, Vivancos A, Conte PF, Guarneri V, Vittoria Dieci M, Griguolo G, Cortés J, Llombart-Cussac A, Muñoz M, Vidal M, Adamo B, Wolff AC, DeMichele A, Villagrasa P, Parker JS, Perou CM, Fernandez-Martinez A, Carey LA, Mittendorf EA, Martín M, Prat A, Tolaney SM. Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer. Ann Oncol. 2023 Sep;34(9):783-795.
- Serrano C, Rothschild S, Villacampa G, Heinrich MC, George S, Blay JY, Sicklick JK, Schwartz GK, Rastogi S, Jones RL, Rutkowski P, Somaiah N, Navarro V, Evans D, Trent JC. Rethinking placebos: embracing synthetic control arms in clinical trials for rare tumors. Nat Med. 2023 Nov;29(11):2689-2692.
- Castelo-Branco L, Pellat A, Martins-Branco D, Valachis A, Derksen JWG, Suijkerbuijk KPM, Dafni U, Dellaporta T, Vogel A, Prelaj A, Groenwold RHH, Martins H, Stahel R, Bliss J, Kather J, Ribelles N, Perrone F, Hall PS, Dienstmann R, Booth CM, Pentheroudakis G, Delaloge S, Koopman M. ESMO Guidance for Reporting Oncology real-World evidence (GROW). Ann Oncol. 2023 Dec;34(12):1097-1112.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 Feb;3(2):251-261.
- Verdaguer H, Saurí T, Acosta DA, Guardiola M, Sierra A, Hernando J, Nuciforo P, Miquel JM, Molero C, Peiró S, Serra-Camprubí Q, Villacampa G, Aguilar S, Vivancos A, Tabernero J, Dienstmann R, Macarulla T. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin Cancer Res. 2022 Apr 14;28(8):1662-1671.
- Villacampa G, Tolosa P, Salvador F, Sánchez-Bayona R, Villanueva L, Dienstmann R, Ciruelos E, Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102352.
- Iacoboni G, Rejeski K, Villacampa G, van Doesum JA, Chiappella A, Bonifazi F, Lopez-Corral L, van Aalderen M, Kwon M, Martínez-Cibrian N, Bramanti S, Reguera-Ortega JL, Camacho-Arteaga L, Schmidt C, Marín-Niebla A, Kersten MJ, Martin Garcia-Sancho A, Zinzani PL, Corradini P, van Meerten T, Subklewe M, Barba P. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2022 Jun 28;6(12):3606-3610.
- Dienstmann R, Tamborero D. Genomic testing for targeted oncology drugs: hopes against hype. Ann Oncol. 2021 Jul;32(7):837-838.
- Villacampa G, Dienstmann R, Bosch F, Abrisqueta P. Combination of novel molecular targeted agent plus R-CHOP-based regimen versus R-CHOP alone in previously untreated diffuse large B-cell lymphoma (DLBCL) patients: a systematic review and meta-analysis. Ann Hematol. 2021 Dec;100(12):2969-2978.
- Matos I, Villacampa G, Hierro C, Martin-Liberal J, Berché R, Pedrola A, Braña I, Azaro A, Vieito M, Saavedra O, Gardeazabal I, Hernando-Calvo A, Alonso G, Galvao V, Ochoa de Olza M, Ros J, Viaplana C, Muñoz-Couselo E, Elez E, Rodon J, Saura C, Macarulla T, Oaknin A, Carles J, Felip E, Tabernero J, Dienstmann R, Garralda E. Phase I prognostic online (PIPO): A web tool to improve patient selection for oncology early phase clinical trials. Eur J Cancer. 2021 Sep;155:168-178.
- Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, Zahm DM, Herencia-Ropero A, Jank P, van Mackelenbergh M, Fasching PA, Marmé F, Stickeler E, Schem C, Dienstmann R, Florian S, Nekljudova V, Balmaña J, Hahnen E, Denkert C, Serra V. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): analysis of the GeparSixto randomized clinical trial. Ann Oncol. 2021 Dec;32(12):1590-1596.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Baird R, Braña I, De Petris L, Yachnin J,Massard C, Opdam FL, Schlenk R, Vernieri C, Garralda E, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium, Calvo F, Fröhling S, Eggermont A, Apolone G, Voest EE, Caldas C, Tabernero J, Ernberg I, Rodon J, Lehtiö J. Support systems to guide clinical decision-making in precision oncology: The Cancer Core Europe Molecular Tumor Board Portal. Nat Med. 2020;26(7):992-994.
- Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I,Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;S0923- 7534(20):39971-3.
- Wagner AH, Walsh B, Mayfield G, Tamborero D, Sonkin D, Krysiak K, Deu-Pons J, Duren RP, Gao J, McMurry J, Patterson S, Del Vecchio Fitz C, Pitel BA, Sezerman OU, Ellrott K, Warner JL, Rieke DT, Aittokallio T, Cerami E, Ritter DI, Schriml LM, Freimuth RR, Haendel M, Raca G, Madhavan S, Baudis M, Beckmann JS, Dienstmann R, Chakravarty D, Li XS, Mockus S, Elemento O, Schultz N, Lopez- Bigas N, Lawler M, Goecks J, Griffith M, Griffith OL, Margolin AA; Variant Interpretation for Cancer Consortium. A harmonized meta-knowledgebase of clinical interpretations of somatic genomic variants in cancer. Nat Genet. 2020;52(4):448-457.
- Dienstmann R, Garralda E, Aguilar S, Sala G, Viaplana C, Ruiz-Pace F, González-Zorelle J, Grazia LoGiacco D, Ogbah Z, Ramos Masdeu L, Mancuso F, Fasani R, Jimenez J, Martinez P, Oaknin A, Saura C, Oliveira M, Balmaña J, Carles J, Macarulla T, Elez E, Alsina M, Braña I, Felip E, Tabernero J, Rodon J, Nuciforo P, Vivancos A. Evolving Landscape of Molecular Prescreening Strategies for Oncology Early Clinical Trials. JCO Precis Oncol. 2020 May 14;4:PO.19.00398.
- Dienstmann R, Villacampa G, Sveen A, Mason MJ, Niedzwiecki D, Nesbakken A, Moreno V, Warren RS, Lothe RA, Guinney J. Relative contribution of clinicopathological variables, genomic markers, transcriptomic subtyping and microenvironment features for outcome prediction in stage II/III colorectal cancer. Ann Oncol. 2019 Oct 1;30(10):1622-1629.
- Dienstmann R, Connor K, Byrne AT; COLOSSUS Consortium. Precision Therapy in RAS Mutant Colorectal Cancer. Gastroenterology 2020;158(4):806-811.
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;7(2):79-92.
- Dienstmann R, Mason MJ, Sinicrope FA, Phipps AI, Tejpar S, Nesbakken A, Danielsen SA, Sveen A, Buchanan DD, Clendenning M, Rosty C, Bot B, Alberts SR, Milburn Jessup J, Lothe RA, Delorenzi M, Newcomb PA, Sargent D, Guinney J. Prediction of overall survival in stage II and III colon cancer beyond the American Joint Commission on Cancer TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28(5):1023-1031.
- Dienstmann R, Elez E, Argiles G, Matos I, Sanz-Garcia E, Ortiz C, Macarulla T, Capdevila J, Alsina M, Sauri T, Verdaguer H, Vilaro M, Ruiz-Pace F, Viaplana C, Garcia A, Landolfi S, Palmer HG, Nuciforo P, Rodon J, Vivancos A, Tabernero J. Analysis of mutant allele fractions in driver genes in colorectal cancer – biological and clinical insights. Mol Oncol. 2017;11(9):1263-1272.
- Dienstmann R, Tabernero J. Cancer: A precision approach to tumor treatment. Nature. 2017 Aug 3;548(7665):40-41.
- Dienstmann R, Jang IS, Bot B, Friend S, Guinney J. Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Cancer Discov 2015 Feb; 5(2): 118-23
- Dienstmann R, Patnaik A, Garcia-Carbonero R, Cervantes A, Benavent M, Roselló S, Tops BB, van der Post RS, Argiles G, Skartved NJ, Hansen UH, Hald R, Pedersen MW, Kragh M, Horak ID, Braun S, Van Cutsem E, Tolcher AW, Tabernero J. Safety and Activity of the First-in-Class Sym004 Anti-EGFR Antibody Mixture in Patients with Refractory Colorectal Cancer. Cancer Discov 2015 Jun; 5(6): 598-609
- Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015 Nov; 21(11): 1350-6
Totes les publicacions
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High FGFR1-4 mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149.
- Pedrola A, Franch-Expósito S, Lahoz S, Esteban-Fabró R, Dienstmann R, Bassaganyas L, Camps J. PCIG: a web-based application to explore immune-genomics interactions across cancer types. Bioinformatics. 2022 Apr 12;38(8):2374-2376.
- Villacampa G, Tolosa P, Salvador F, Sánchez-Bayona R, Villanueva L, Dienstmann R, Ciruelos E, Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102352.
- Verdaguer H, Saurí T, Acosta DA, Guardiola M, Sierra A, Hernando J, Nuciforo P, Miquel JM, Molero C, Peiró S, Serra-Camprubí Q, Villacampa G, Aguilar S, Vivancos A, Tabernero J, Dienstmann R, Macarulla T. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin Cancer Res. 2022 Apr 14;28(8):1662-1671.
- Villacampa G, Tolosa P, Salvador F, Sánchez-Bayona R, Villanueva L, Dienstmann R, Ciruelos E, Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102352.
- Pellegrino B, Herencia-Ropero A, Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C, Villacampa G, Guzmán M, Rodríguez O, Grueso J, Jiménez J, Arenas EJ, Degasperi A, Dias JML, Forment JV, O’Connor MJ, Déas O, Cairo S, Zhou Y, Musolino A, Caldas C, Nik-Zainal S, Clarke RB, Nuciforo P, Díez O, Serres-Créixams X, Peg V, Espinosa-Bravo M, Macarulla T, Oaknin A, Mateo J, Arribas J, Dienstmann R, Bellet M, Oliveira M, Saura C, Gutiérrez-Enríquez S, Balmaña J, Serra V. Preclinical In Vivo Validation of the RAD51 Test for Identification of Homologous Recombination-Deficient Tumors and Patient Stratification. Cancer Res. 2022 Apr 15;82(8):1646-1657.
- Tamborero D, Dienstmann R, Rachid MH, Boekel J, Lopez-Fernandez A, Jonsson M, Razzak A, Braña I, De Petris L, Yachnin J, Baird RD, Loriot Y, Massard C, Martin-Romano P, Opdam F, Schlenk RF, Vernieri C, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium; Balmaña J, Apolone G, Caldas C, Bergh J, Ernberg I, Fröhling S, Garralda E, Karlsson C, Tabernero J, Voest E, Rodon J, Lehtiö J. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer. 2022 Feb;3(2):251-261.
- Palafox M, Monserrat L, Bellet M, Villacampa G, Gonzalez-Perez A, Oliveira M, Brasó-Maristany F, Ibrahimi N, Kannan S, Mina L, Herrera-Abreu MT, Òdena A, Sánchez-Guixé M, Capelán M, Azaro A, Bruna A, Rodríguez O, Guzmán M, Grueso J, Viaplana C, Hernández J, Su F, Lin K, Clarke RB, Caldas C, Arribas J, Michiels S, García-Sanz A, Turner NC, Prat A, Nuciforo P, Dienstmann R, Verma CS, Lopez-Bigas N, Scaltriti M, Arnedos M, Saura C, Serra V. High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER+ breast cancer. Nat Commun. 2022 Sep 7;13(1):5258.
- Jácome AA, Peixoto RD, Gil MV, Ominelli J, Prolla G, Dienstmann R, Eng C. Biologics in rectal cancer. Expert Opin Biol Ther. 2022 Oct;22(10):1245-1257.
- Biologics in rectal cancer.
- Dienstmann R, Lonardi S. Is upfront full molecular profiling needed in all patients with colorectal cancer in daily practice? Lancet Oncol. 2022 Sep;23(9):1129-1131.
- Le Tourneau C, Perret C, Hackshaw A, Blay JY, Nabholz C, Geissler J, Do T, von Meyenn M, Dienstmann R. An Approach to Solving the Complex Clinicogenomic Data Landscape in Precision Oncology: Learnings From the Design of WAYFIND-R, a Global Precision Oncology Registry. JCO Precis Oncol. 2022 Jul;6:e2200019.
- Villacampa G, Hernando-Calvo A, Berché R, Saavedra O, Marmolejo D, Mirallas O, Braña I, Muñoz-Couselo E, Garralda E, Dienstmann R. Response to “Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias”. Cancer Treat Rev. 2022 Dec;111:102465.
- Elez E, Ros J, Fernández J, Villacampa G, Moreno-Cárdenas AB, Arenillas C, Bernatowicz K, Comas R, Li S, Kodack DP, Fasani R, Garcia A, Gonzalo-Ruiz J, Piris-Gimenez A, Nuciforo P, Kerr G, Intini R, Montagna A, Germani MM, Randon G, Vivancos A, Smits R, Graus D, Perez-Lopez R, Cremolini C, Lonardi S, Pietrantonio F, Dienstmann R, Tabernero J, Toledo RA. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat Med. 2022 Oct;28(10):2162-2170.
- Brown S, Lavery JA, Shen R, Martin AS, Kehl KL, Sweeney SM, Lepisto EM, Rizvi H, McCarthy CG, Schultz N, Warner JL, Park BH, Bedard PL, Riely GJ, Schrag D, Panageas KS; AACR Project GENIE Consortium. Implications of Selection Bias Due to Delayed Study Entry in Clinical Genomic Studies. JAMA Oncol. 2022 Feb 1;8(2):287-291.
- Van Egeren D, Kohli K, Warner JL, Bedard PL, Riely G, Lepisto E, Schrag D, LeNoue-Newton M, Catalano P, Kehl KL, Michor F; AACR Project GENIE Consortium represented by Shawn Sweeney. Genomic analysis of early-stage lung cancer reveals a role for TP53 mutations in distant metastasis. Sci Rep. 2022 Nov 9;12(1):19055.
- Duarte FA, Ferreira CG, Dienstmann R, Ferrari BL, Costa E Silva M, Nazareth A Junior P, Guilherme de O Salles P, Henrique C Diniz P. Barriers in precision medicine implementation among Advanced Nonsquamous Cell Lung Cancer-patients: A Real-World Evidence Scenario. J Mark Access Health Policy. 2022 May 24;10(1):2077905.
- Élez E, Mulet-Margalef N, Sanso M, Ruiz-Pace F, Mancuso FM, Comas R, Ros J, Argilés G, Martini G, Sanz-Garcia E, Baraibar I, Salvà F, Noguerido A, Cuadra-Urteaga JL, Fasani R, Garcia A, Jimenez J, Aguilar S, Landolfi S, Hernández-Losa J, Braña I, Nuciforo P, Dienstmann R, Tabernero J, Salazar R, Vivancos A. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy. Int J Mol Sci. 2022 Dec 21;24(1):118. A Comprehensive Biomarker Analysis of Microsatellite Unstable/Mismatch Repair Deficient Colorectal Cancer Cohort Treated with Immunotherapy.
- Resende CAA, Fernandes Cruz HM, Costa E Silva M, Paes RD, Dienstmann R, Barrios CHE, Goncalves AC, Cascelli FGA, Souto AKBA, Oliveira LC, Reinert T, Andrade DAP, Passos MP, Millen EC, Zerwes F, Moraes PL, Ferrari BL, Mano MS. Impact of the COVID-19 Pandemic on Cancer Staging: An Analysis of Patients With Breast Cancer From a Community Practice in Brazil. JCO Glob Oncol. 2022 Nov;8:e2200289.
- Iacoboni G, Rejeski K, Villacampa G, van Doesum JA, Chiappella A, Bonifazi F, Lopez-Corral L, van Aalderen M, Kwon M, Martínez-Cibrian N, Bramanti S, Reguera-Ortega JL, Camacho-Arteaga L, Schmidt C, Marín-Niebla A, Kersten MJ, Martin Garcia-Sancho A, Zinzani PL, Corradini P, van Meerten T, Subklewe M, Barba P. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2022 Jun 28;6(12):3606-3610.
- Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, Espinosa-Bravo M, Peg V, Vivancos A, Saura C, Villacampa G, Oliveira M. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev. 2022 Mar;104:102362.
- Remon J, Hendriks LEL, Mountzios G, García-Campelo R, Saw SPL, Uprety D, Recondo G, Villacampa G, Reck M. MET alterations in NSCLC-Current Perspectives and Future Challenges. J Thorac Oncol. 2022 Oct 29:S1556-0864(22)01865-2.
- Prat A, Paz-Ares L, Juan M, Felip E, Garralda E, González B, Arance A, Martín-Liberal J, Gavilá J, López-González A, Cejalvo JM, Izarzugaza Y, Amillano K, Corbacho JG, Saura C, Racca F, Hierro C, Sanfeliu E, Gonzalez X, Canes J, Villacampa G, Salvador F, Pascual T, Mesía R, Cervantes A, Tabernero J. SOLTI-1904 ACROPOLI TRIAL: efficacy of spartalizumab monotherapy across tumor-types expressing high levels of PD1 mRNA. Future Oncol. 2022 Oct 6.
- Jiménez M, Roldán E, Fernández-Naval C, Villacampa G, Martinez-Gallo M, Medina-Gil D, Peralta-Garzón S, Pujadas G, Hernández C, Pagès C, Gironella M, Fox L, Orti G, Barba P, Pumarola T, Cabirta A, Catalá E, Valentín M, Marín-Niebla A, Orfao A, González M, Campins M, Ruiz-Camps I, Valcárcel D, Bosch F, Hernández M, Crespo M, Esperalba J, Abrisqueta P. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022 Feb 8;6(3):774-784.
- Zurita AJ, Graf RP, Villacampa G, Raskina K, Sokol E, Jin D, Antonarakis ES, Li G, Huang RSP, Casanova-Salas I, Vivancos A, Carles J, Ross JS, Schrock AB, Oxnard GR, Mateo J. Genomic Biomarkers and Genome-Wide Loss-of-Heterozygosity Scores in Metastatic Prostate Cancer Following Progression on Androgen-Targeting Therapies. JCO Precis Oncol. 2022 Jul;6:e2200195.
- Abrisqueta P, Medina D, Villacampa G, Lu J, Alcoceba M, Carabia J, Boix J, Tazón-Vega B, Iacoboni G, Bobillo S, Marín-Niebla A, González M, Zenz T, Crespo M, Bosch F. A gene expression assay based on chronic lymphocytic leukemia activation in the microenvironment to predict progression. Blood Adv. 2022 Nov 8;6(21):5763-5773.
- Villacampa G, Falato C, Paré L, Hernando C, Arumí M, Saura C, Gómez G, Muñoz M, Gil-Gil M, Izarzugaza Y, Ferrer N, Najera-Zuloaga J, Montaño A, Ciruelos E, González-Santiago S, Villagrasa P, Gavilá J, Prat A, Pascual T. Pre-operative ribociclib plus letrozole versus chemotherapy: Health-related quality of life outcomes from the SOLTI CORALLEEN trial. Eur J Cancer. 2022 Oct;174:232-242.
- Vila CM, Moreno FA, Estébanez MM, Ares GR, Villacampa G, Dashti P, Oberoi HS, Martin-Huertas R, Jares P, Alos L, Teixido C, Rull R, Sanchez M, Malvehy J, Carcelero E, Valduvieco I, Fernandez AA. Exploratory analysis of clinical benefit of ipilimumab and nivolumab treatment in patients with metastatic melanoma from a single institution. Clin Transl Oncol. 2022 Feb;24(2):319-330.
- Carrasco E, López-Fernández A, Codina-Sola M, Valenzuela I, Cueto-González AM, Villacampa G, Navarro V, Torres-Esquius S, Palau D, Cruellas M, Torres M, Perez-Dueñas B, Abulí A, Diez O, Sábado-Álvarez C, García-Arumí E, Tizzano EF, Moreno L, Balmaña J. Clinical and psychological implications of secondary and incidental findings in cancer susceptibility genes after exome sequencing in patients with rare disorders. J Med Genet. 2022 Nov 29:jmg-2022-108929.
- Garcia-Duran C, Grau F, Villacampa G, Oaknin A. ATOMICC trial: a randomized, open-label, phase II trial of anti-PD1, dostarlimab, as maintenance therapy for patients with high-risk locally advanced cervical cancer after chemoradiation. Int J Gynecol Cancer. 2022 Apr 20:ijgc-2022-003370.
- Ligero M, Garcia-Ruiz A, Viaplana C, Villacampa G, Raciti MV, Landa J, Matos I, Martin-Liberal J, Ochoa-de-Olza M, Hierro C, Mateo J, Gonzalez M, Morales-Barrera R, Suarez C, Rodon J, Elez E, Braña I, Muñoz-Couselo E, Oaknin A, Fasani R, Nuciforo P, Gil D, Rubio-Perez C, Seoane J, Felip E, Escobar M, Tabernero J, Carles J, Dienstmann R, Garralda E, Perez-Lopez R. A CT-based Radiomics Signature Is Associated with Response to Immune Checkpoint Inhibitors in Advanced Solid Tumors. Radiology. 2021 Apr;299(1):109-119.
- Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, Zahm DM, Herencia-Ropero A, Jank P, van Mackelenbergh M, Fasching PA, Marmé F, Stickeler E, Schem C, Dienstmann R, Florian S, Nekljudova V, Balmaña J, Hahnen E, Denkert C, Serra V. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): analysis of the GeparSixto randomized clinical trial. Ann Oncol. 2021 Dec;32(12):1590-1596.
- Dienstmann R, Tamborero D. Genomic testing for targeted oncology drugs: hopes against hype. Ann Oncol. 2021 Jul;32(7):837-838.
- Kulkarni AA, Naqash AR, Puri S, Dienstmann R. Is It Time to Implement Adjuvant Targeted Therapy in EGFR-Mutant Non-Small-Cell Lung Cancer? JCO Precis Oncol. 2021 Feb 17;5:PO.20.00460
- Ros J, Baraibar I, Martini G, Salvà F, Saoudi N, Cuadra-Urteaga JL, Dienstmann R, Tabernero J, Élez E. The Evolving Role of Consensus Molecular Subtypes: a Step Beyond Inpatient Selection for Treatment of Colorectal Cancer. Curr Treat Options Oncol. 2021 Nov 6;22(12):113.
- Werutsky G, Barrios CH, Cardona AF, Albergaria A, Valencia A, Ferreira CG, Rolfo C, de Azambuja E, Rabinovich GA, Sposetti G, Arrieta O, Dienstmann R, Rebelatto TF, Denninghoff V, Aran V, Cazap E. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021 Nov;22(11):e488-e500.
- Gris-Oliver A, Ibrahim YH, Rivas MA, García-García C, Sánchez-Guixé M, Ruiz-Pace F, Viaplana C, Pérez-García JM, Llombart-Cussac A, Grueso J, Parés M, Guzmán M, Rodríguez O, Anton P, Cozar P, Calvo MT, Bruna A, Arribas J, Caldas C, Dienstmann R, Nuciforo P, Oliveira M, Cortés J, Serra V. PI3K activation promotes resistance to eribulin in HER2-negative breast cancer. Br J Cancer. 2021 Apr;124(9):1581-1591.
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High FGFR1-4 mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149.
- Hernando-Calvo A, García-Alvarez A, Villacampa G, Ortiz C, Bodet D, García-Patos V, Recio JA, Dienstmann R, Muñoz-Couselo E. Dynamics of clinical biomarkers as predictors of immunotherapy benefit in metastatic melanoma patients. Clin Transl Oncol. 2021 Feb;23(2):311-317.
- Saura C, Sánchez O, Martínez S, Domínguez C, Dienstmann R, Ruíz-Pace F, Céspedes MC, Peñuelas Á, Cortés J, Llurba E, Córdoba O. Evolution of Angiogenic Factors in Pregnant Patients with Breast Cancer Treated with Chemotherapy. Cancers (Basel). 2021 Feb 23;13(4):923.
- De Mattos-Arruda L, Cortes J, Blanco-Heredia J, Tiezzi DG, Villacampa G, Gonçalves-Ribeiro S, Paré L, Souza CA, Ortega V, Sammut SJ, Cusco P, Fasani R, Chin SF, Perez-Garcia J, Dienstmann R, Nuciforo P, Villagrasa P, Rubio IT, Prat A, Caldas C. The temporal mutational and immune tumour microenvironment remodelling of HER2-negative primary breast cancers. NPJ Breast Cancer. 2021 Jun 7;7(1):73.
- Cedres S, Assaf JD, Iranzo P, Callejo A, Pardo N, Navarro A, Martinez-Marti A, Marmolejo D, Rezqallah A, Carbonell C, Frigola J, Amat R, Pedrola A, Dienstmann R, Felip E. Efficacy of chemotherapy for malignant pleural mesothelioma according to histology in a real-world cohort. Sci Rep. 2021 Nov 1;11(1):21357.
- Matos I, Villacampa G, Hierro C, Martin-Liberal J, Berché R, Pedrola A, Braña I, Azaro A, Vieito M, Saavedra O, Gardeazabal I, Hernando-Calvo A, Alonso G, Galvao V, Ochoa de Olza M, Ros J, Viaplana C, Muñoz-Couselo E, Elez E, Rodon J, Saura C, Macarulla T, Oaknin A, Carles J, Felip E, Tabernero J, Dienstmann R, Garralda E. Phase I prognostic online (PIPO): A web tool to improve patient selection for oncology early phase clinical trials. Eur J Cancer. 2021 Sep;155:168-178.
- Lafferty A, O’Farrell AC, Migliardi G, Khemka N, Lindner AU, Sassi F, Zanella ER, Salvucci M, Vanderheyden E, Modave E, Boeckx B, Halang L, Betge J, Ebert MPA, Dicker P, Argilés G, Tabernero J, Dienstmann R, Medico E, Lambrechts D, Bertotti A, Isella C, Trusolino L, Prehn JHM, Byrne AT. Molecular Subtyping Combined with Biological Pathway Analyses to Study Regorafenib Response in Clinically Relevant Mouse Models of Colorectal Cancer. Clin Cancer Res. 2021 Nov 1;27(21):5979-5992.
- Ligero M, Jordi-Ollero O, Bernatowicz K, Garcia-Ruiz A, Delgado-Muñoz E, Leiva D, Mast R, Suarez C, Sala-Llonch R, Calvo N, Escobar M, Navarro-Martin A, Villacampa G, Dienstmann R, Perez-Lopez R. Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Eur Radiol. 2021 Mar;31(3):1460-1470.
- Morgan G, Tagliamento M, Lambertini M, Devnani B, Westphalen B, Dienstmann R, Bozovic-Spasojevic I, Calles A, Criscitiello C, Curioni A, Garcia AM, Lamarca A, Pilotto S, Scheffler M, Strijbos M, Wong R, de Azambuja E, Peters S. Impact of COVID-19 on social media as perceived by the oncology community: results from a survey in collaboration with the European Society for Medical Oncology (ESMO) and the OncoAlert Network. ESMO Open. 2021 Apr;6(2):100104.
- Kehl KL, Riely GJ, Lepisto EM, Lavery JA, Warner JL, LeNoue-Newton ML, Sweeney SM, Rudolph JE, Brown S, Yu C, Bedard PL, Schrag D, Panageas KS; American Association of Cancer Research (AACR) Project Genomics Evidence Neoplasia Information Exchange (GENIE) Consortium. Correlation Between Surrogate End Points and Overall Survival in a Multi-institutional Clinicogenomic Cohort of Patients With Non-Small Cell Lung or Colorectal Cancer. JAMA Netw Open. 2021 Jul 1;4(7):e2117547.
- Villacampa G, Dienstmann R, Bosch F, Abrisqueta P. Combination of novel molecular targeted agent plus R-CHOP-based regimen versus R-CHOP alone in previously untreated diffuse large B-cell lymphoma (DLBCL) patients: a systematic review and meta-analysis. Ann Hematol. 2021 Dec;100(12):2969-2978.
- López-Fernández A, Villacampa G, Grau E, Salinas M, Darder E, Carrasco E, Torres-Esquius S, Iglesias S, Solanes A, Gadea N, Velasco A, Urgell G, Torres M, Tuset N, Brunet J, Corbella S, Balmaña J. Patients’ and professionals’ perspective of non-in-person visits in hereditary cancer: predictors and impact of the COVID-19 pandemic. Genet Med. 2021 Aug;23(8):1450-1457.
- Iacoboni G, Simó M, Villacampa G, Catalá E, Carpio C, Díaz-Lagares C, Vidal-Jordana Á, Bobillo S, Marín-Niebla A, Pérez A, Jiménez M, Abrisqueta P, Bosch F, Barba P. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy. Ann Hematol. 2021 Sep;100(9):2303-2310.
- Ortí G, Palacio-Garcia C, García-Cadenas I, Sánchez-Ortega I, Jimenez MJ, Azqueta C, Villacampa G, Ferrà C, Parody R, Martino R, Bosch F, Querol S, Valcárcel D. Analysis of Cell Subsets in Donor Lymphocyte Infusions from HLA Identical Sibling Donors after Allogeneic Hematopoietic Cell Transplant. Transplant Cell Ther. 2021 Jan;27(1):53.e1-53.e8.
- Quintana Á, Peg V, Prat A, Moliné T, Villacampa G, Paré L, Galván P, Dientsmann R, Schmid P, Curigliano G, Muñoz-Couselo E, Perez-García J, Marti M, Blanco-Heredia J, Anjos CD, Vazquez M, De Mattos-Arruda L, Cortés J. Immune analysis of lymph nodes in relation to the presence or absence of tumor infiltrating lymphocytes in triple-negative breast cancer. Eur J Cancer. 2021 May;148:134-145.
- Elez E, Ayala F, Felip E, García Campelo R, García Carbonero R, García Donás J, González Del Alba A, González Flores E, Hidalgo J, Isla D, Majem M, Rodríguez Lescure Á, Safont MJ, Santaballa A, Villacampa G, Vera R, Garrido P. Gender influence on work satisfaction and leadership for medical oncologists: a survey of the Spanish Society of Medical Oncology (SEOM). ESMO Open. 2021 Apr;6(2):100048.
- Pascual T, Oliveira M, Ciruelos E, Bellet Ezquerra M, Saura C, Gavilá J, Pernas S, Muñoz M, Vidal MJ, Margelí Vila M, Cejalvo JM, González-Farré B, Espinosa-Bravo M, Cruz J, Salvador-Bofill FJ, Guerra JA, Luna Barrera AM, Arumi de Dios M, Esker S, Fan PD, Martínez-Sáez O, Villacampa G, Paré L, Ferrero-Cafiero JM, Villagrasa P, Prat A. SOLTI-1805 TOT-HER3 Study Concept: A Window-of-Opportunity Trial of Patritumab Deruxtecan, a HER3 Directed Antibody Drug Conjugate, in Patients With Early Breast Cancer. Front Oncol. 2021 Apr 23;11:638482.
- Carpio C, Iacoboni G, Villacampa G, Catalá E, Bobillo S, Pérez A, Jiménez M, Segura L, Olivé M, Farriols A, Abrisqueta P, Valcárcel D, Carreras MJ, Bosch F, Barba P. Selection process and causes of non-eligibility for CD19 CAR-T cell therapy in patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma in a European center. Leuk Lymphoma. 2021 Sep;62(9):2288-2291.
- Vila CM, Moreno FA, Estébanez MM, Ares GR, Villacampa G, Dashti P, Oberoi HS, Martin-Huertas R, Jares P, Alos L, Teixido C, Rull R, Sanchez M, Malvehy J, Carcelero E, Valduvieco I, Fernandez AA. Exploratory analysis of clinical benefit of ipilimumab and nivolumab treatment in patients with metastatic melanoma from a single institution. Clin Transl Oncol. 2022 Feb;24(2):319-330.
- Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, Guerreiro M, Caballero AC, Mussetti A, Sancho JM, Hernani R, Abrisqueta P, Solano C, Sureda A, Briones J, Martin Garcia-Sancho A, Kwon M, Reguera-Ortega JL, Barba P; GETH, GELTAMO Spanish Groups. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021 May;10(10):3214-3223.
- Evidence-based neoadjuvant endocrine therapy for breast cancer. Dienstmann R, Bines J. Clin Breast Cancer 2006;7(4):315-20.
- Phase II trial of neoadjuvant chemotherapy using alternating doublets in non-small cell lung cancer. Martins RG, Dienstmann R, Biasi P, Dantas K, Santos VO, Toscano E, Roriz W, Zamboni M, Sousa A, Ferreira CG, Small I, Moreira D, Zukin M. Clin Lung Cancer 2007;8(4):257-63.
- Phase II trial of neoadjuvant gemcitabine and cisplatin in patients with invasive bladder carcinoma. Herchenhorn D, Dienstmann R, Peixoto FA, Campos FS, Santos VO, Moreira DM, Cardoso H, Small I, Ferreira CG. Int Braz J Urol 2007;33(5):630-8.
- Palliative percutaneous nephrostomy in recurrent cervical cancer: Retrospective analysis of 50 consecutive cases Dienstmann R, Pinto CS, Pereira MT, Small I, Ferreira CG. J Pain Symptom Manage 2008;36(2):185-90.
- XAF1 mRNA expression improves progression-free and overall survival for patients with advanced bladder cancer treated with neoadjuvant chemotherapy Pinho MB, Costas F, Sellos J, Dienstmann R, Andrade PB, Herchenhorn D, Peixoto FA, Santos VO, Small IA, Guimarães DP, Ferreira CG. Urol Oncol 2009;27(4):382-90.
- Necitumumab (IMC-11F8), a fully human IgG1 mAb directed against epidermal growth factor receptor for the intravenous treatment of cancer. Dienstmann R, Tabernero J. Curr Opin Investig Drugs 2010;11(12):1434-41.
- Recent developments in anti-cancer Agents targeting PI3K, Akt and mTORC1/2. Dienstmann R, Rodon J, Tabernero J. Recent Pat Anticancer Drug Discov 2011;6(2):210-36.
- Targeting the PI3K-Akt-mTOR pathway – beyond rapalogs. Markman B, Dienstmann R, Tabernero J. Oncotarget 2010;1(7):530-43.
- BRAF as a target for cancer therapy. Dienstmann R, Tabernero J. Anticancer Agents Med Chem 2011;11(3):285-95.
- Molecular predictors of response to chemotherapy in colorectal cancer. Dienstmann R, Vilar E, Tabernero J. Cancer J 2011;17(2):114-26.
- Personalizing therapy with targeted agents in non-small cell lung cancer. Dienstmann R, Martinez P, Felip E. Oncotarget 2011;2(3):165-77.
- Combined modality therapy of stage IIIC breast cancer. Dienstmann R, Branco LG, Rezende LM, Freitas LC, Lima CF, Rodrigues GJ, Noronha Filho H, Sarmento RMB, Small I, Bines J. Breast J 2011;17(3):331-3.
- Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Dienstmann R, Felip E. Expert Opin Biol Ther 2011;11(9):1223-31.
- The development of molecular biomarkers in the individualized treatment of colorectal cancer. De Mattos-Arruda L, Dienstmann D, Tabernero J. Clin Colorectal Cancer 2011;10(4):279-89.
- Risk-benefit assessment of bevacizumab in the treatment of breast cancer. Dienstmann R, Ades F, Saini KS, Metzger-Filho O. Drug Saf 2012;35(1):15-25.
- Toxicity as biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGFR inhibiting anticancer agents. Dienstmann R, Braña I, Rodon J, Tabernero J. Oncologist 2011;16(12):1729-40.
- Drug development to overcome resistance to EGFR inhibitors in lung and colorectal cancer. Dienstmann R, De Dosso S, Felip E, Tabernero J. Mol Oncol 2012;6(1):15-26.
- Application of monoclonal antibodies as cancer therapy in solid tumors. Dienstmann R, Markman B, Tabernero J. Curr Clin Pharmacol 2012;7(2):137-45.
- Panitumumab: summary of clinical development in colorectal cancer and future directions. Argiles G, Dienstmann R, Tabernero J. Future Oncol 2012;8(4):373-89.
- Molecular prescreening to select patient population in early clinical trials. Rodón J, Saura C, Dienstmann R, Vivancos A, Cajal SR, Baselga J, Tabernero J. Nat Rev Clin Oncol 2012;9(6):359-66
- Molecular profiling of patients with colorectal cancer and matched targeted therapy in Phase 1 clinical trials. Dienstmann R, Serpico D, Rodon J, Saura C, Macarulla T, Elez ME, Alsina M, Capdevila J, Perez-Garcia J, Sánchez-Ollé G, Aura C, Prudkin L, Landolfi S, Hernández-Losa J, Vivancos A, Tabernero J. Mol Cancer Ther 2012;11(9):2062-71.
- Drug development in the era of personalized oncology: from population-based trials to enrichment and prescreening strategies. Dienstmann R, Rodon J, Tabernero J. Am Soc Clin Oncol Educ Book 2012;32:168-72.
- Development of PI3K inhibitors: lessons learned from early clinical trials. Rodon J, Dienstmann R, Serra V, Tabernero J. Nat Rev Clin Oncol 2013;10(3):143-53.
- Biomarker-driven patient selection for early clinical trials. Dienstmann R, Rodon J, Barretina J, Tabernero J. Curr Opin Oncol 2013;25(3):305-12.
- The genomic medicine frontier in human solid tumors: prospects and challenges. Dienstmann R, Rodon J, Barretina J, Tabernero J. J Clin Oncol 2013;31(15):1874-84.
- Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Annals Oncol 2014;25(3):552-63.
- Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial. Bines J, Dienstmann R, Obadia RM, Branco LGB, Quintella DC, Castro TM, Camacho PG, Soares F, Costa MEF. Annals Oncol 2014;25(4):831-6.
- Tumor Growth Rate (TGR) analysis in Phase I clinical trials–Letter. Dienstmann R, Tabernero J. Clin Cancer Res 2014;20(9):2495-6.
- Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Dienstmann R, Rodon J, Serra V, Tabernero J. Mol Cancer Ther 2014;13(5):1021-31.
- Standardized decision support in sequencing reports of somatic cancer variants. Dienstmann R, Dong F, Borger D, Dias-Santagata D, Ellisen LW, Le LP, Iafrate AJ. Mol Oncol 2014;8(5):859-873.
- The evolution of our molecular understanding of colorectal cancer: what we are doing now, what the future holds, and how tumor profiling is just the beginning. Dienstmann R, Salazar R, Tabernero J. Am Soc Clin Oncol Educ Book 2014;34:91-9.
- Optimal design of trials to demonstrate the utility of genomically-guided therapy: putting Precision Cancer Medicine to the test. Dienstmann R, Rodon J, Tabernero J. Mol Oncol 2014;S1574-7891(14)00150-1.
- Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dienstmann R, Delmonte A, Cereda R, Isaacson J, Litten J, Allen A, Dubois F, Saba C, Robert R, D’Incalci M, Zucchetti M, Camboni MG, Tabernero J. Ann Oncol 2014;25(11):2244-51.
- Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M, Goldberg RM, Mahoney M, Sargent DJ, Alberts SR. Gastroenterology 2015;148(1):88-99.
- Social interactomes for enabling research communities. Guinney J, Dienstmann R, Ferté C, Friend S, McCormick F. Cancer Discov 2014;4(11):1265-8.
- Stepwise group sparse regression (SGSR): gene-set-based pharmacogenomic predictive models with stepwise selection of functional priors. Jang IS, Dienstmann R, Margolin AA, Guinney J. Pac Symp Biocomput 2015;20:32-43.
- Intrinsic cancer subtypes – next steps into personalized medicine. Santos C, Sanz-Pamplona R, Nadal E, Grasselli J, Pernas S, Dienstmann R, Moreno V, Tabernero J, Salazar R. Cell Oncol (Dordr) 2015;38(1):3-16.
- Genomic testing in colorectal cancer: how much is enough? Dienstmann R, Salazar R, Tabernero J. Oncology (Williston Park) 2015;29(3):186-8.
- A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Mau-Sørensen M, Dittrich C, Dienstmann R, Lassen U, Büchler W, Martinius H, Tabernero J. Cancer Chemother Pharmacol 2015;75(5):1065-73.
- Database of genomic biomarkers for cancer drugs and clinical targetability in solid tumors. Dienstmann R, Jang IS, Bot B, Friend S, Guinney J. Cancer Discovery 2015;5(2):118-23.
- Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. Dienstmann R, Salazar R, Tabernero J. J Clin Oncol 2015; 33(16):1787-96.
- Safety and pharmacokinetics/pharmacodynamics of the first-in-class dual action HER3/EGFR antibody MEHD7945A in patients with locally advanced or metastatic epithelial tumors. Juric D, Dienstmann R, Cervantes A, Hidalgo M, Messersmith W, Blumenschein Jr. GR, Tabernero J, Roda D, Calles A, Jimeno A, Wang A, Bohórquez SS, Leddy C, Littman C, Kapp AV, Shames DS, Penuel E, Amler LC, Pirzkall A, Baselga J. Clin Cancer Res 2015;21(11):2462-70.
- Heterogeneity of driver genes and therapeutic implications in colorectal cancer. Dienstmann R, Cervantes A. Annals Oncol 2015;26(8):1523-5.
- Overcoming Resistance to anti-EGFR Therapy in Colorectal Cancer. Dienstmann R, Salazar R, Tabernero J. Am Soc Clin Oncol Educ Book 2015;35:e149-56.
- Safety and activity of the first-in-class Sym004 anti-EGFR antibody mixture in patients with refractory colorectal cancer. Dienstmann R, Patnaik A, Garcia-Carbonero R, Cervantes A, Benavent M, Roselló S, Tops BB, van der Post RS, Argilés G, Skartved NJ, Hansen UH, Hald R, Pedersen MW, Kragh M, Horak ID, Braun S, Van Cutsem E, Tolcher AW, Tabernero J. Cancer Discov 2015;5(6):598-609.
- Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced solid tumors. Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, Calvo E, Moreno V, Adamo B, Gazzah A, Zhong B, Platero SJ, Smit H, Stuyckens K, Chatterjee-Kishore M, Rodon J, Peddareddigari V, Luo FR, Soria J-C. J Clin Oncol 2015;33(30):3401-8.
- The consensus molecular subtypes of colorectal cancer. Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. Nat Med 2015;21(11):1350-6.
- MEK plus PI3K/mTORC1/2 therapeutic efficacy is impacted by TP53 mutation in preclinical models of colorectal cancer. García-García C, Rivas MA, Ibrahim YH, Calvo MT, Gris-Oliver A, Rodríguez O, Grueso J, Antón P, Guzmán M, Aura C, Nuciforo P, Jessen K, Argilés G, Dienstmann R, Bertotti A, Trusolino L, Matito J, Vivancos A, Chicote I, Palmer HG, Tabernero J, Scaltriti M, Baselga J, Serra V. Clin Cancer Res 2015;21(24):5499-510.
- Tankyrase inhibition blocks Wnt/β-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Arques O, Chicote I, Puig I, Tenbaum SP, Argiles G, Dienstmann R, Fernandez N, Caratu G, Matito J, Silberschmidt D, Rodon J, Landolfi S, Prat A, Espin E, Charco R, Nuciforo P, Vivancos A, Shao W, Tabernero J, Palmer HG. Clin Cancer Res 2016;22(3):644-56.
- First-in-man dose-escalation study of the selective BRAF inhibitor RG7256 in patients with BRAF V600-mutated advanced solid tumors. Dienstmann R, Lassen U, Cebon J, Desai J, Brown MP, Evers S, Su F, Zhang W, Doisserie F, Lestini B, Schostack K, Meresse V, Tabernero J. Target Oncol 2016;11(2):149-56.
- Nanofluidic Digital PCR and Extended Genotyping of RAS and BRAF for Improved Selection of Metastatic Colorectal Cancer Patients for Anti-EGFR Therapies. Azuara D, Santos C, Lopez-Doriga A, Grasselli J, Nadal M, Sanjuan X, Marin F, Vidal J, Montal R, Moreno V, Bellosillo B, Argiles G, Elez E, Dienstmann R, Montagut C, Tabernero J, Capellá G, Salazar R. Mol Cancer Ther 2016;15(5):1106-12.
- Spectrum of Gene Mutations in Colorectal Cancer: Implications for Treatment. Dienstmann R, Tabernero J. Cancer J 2016;22(3):149-55.
- CIViC: A knowledgebase for expert-crowdsourcing the clinical interpretation of variants in cancer. Griffith M, Spies NC, Krysiak K, Coffman AC, McMichael JF, Ainscough BJ, Rieke DJ, Danos AM, Kujan L, Ramirez CA, Wagner AH, Skidmore ZL, Liu CJ, Jones M, Bilski RL, Lesurf R, Barnell EK, Shah NM, Bonakdar M, Trani L, Matlock M, Ramu A, Campbell KM, Spies GC, Graubert AP, Karthik G, Eldred JM, Larson DE, Walker JR, Chu S, Bryan JN, Good BN, Wu C, Su AI, Dienstmann R, Jones SJM, Bose R, Spencer DH, Wartman LD, Wilson RK, Mardis ER, Griffith OL. Nat Genetics 2017;49(2):170-174.
- Consensus Molecular Subtyping and the Evolution of Precision Medicine in Colorectal Cancer. Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Nat Rev Cancer 2017;7(2):79-92.
- Prediction of overall survival in stage II and III colon cancer beyond the American Joint Commission on Cancer TNM system: a retrospective, pooled biomarker study. Dienstmann R, Mason MJ, Sinicrope FA, Phipps AI, Tejpar S, Nesbakken A, Danielsen SA, Sveen A, Buchanan DD, Clendenning M, Rosty C, Bot B, Alberts SR, Milburn Jessup J, Lothe RA, Delorenzi M, Newcomb PA, Sargent D, Guinney J. Ann Oncol 2017;28(5):1023-1031.
- Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, Vidal J, Garcia M, Viéitez JM, Paéz D, Falcó E, Lopez Lopez C, Aranda E, Jones F, Sikri V, Nuciforo P, Fasani R, Tabernero J, Montagut C, Azuara D, Dienstmann R, Salazar R, Vivancos A. Ann Oncol 2017 Jun 1;28(6):1294-1301.
- Pharmacokinetic/Pharmacodynamic Modeling for Drug Development in Oncology. Garralda E, Dienstmann R, Tabernero J. Am Soc Clin Oncol Educ Book 2017;37:210-215.
- Personalizing Adjuvant Therapy for Stage II/III Colorectal Cancer. McCleary NJ, Benson AB 3rd, Dienstmann R. Am Soc Clin Oncol Educ Book 2017;37:232-245.
- Analysis of mutant allele fractions in driver genes in colorectal cancer – biological and clinical insights. Dienstmann R, Elez E, Argiles G, Matos I, Sanz-Garcia E, Ortiz C, Macarulla T, Capdevila J, Alsina M, Sauri T, Verdaguer H, Vilaro M, Ruiz-Pace F, Viaplana C, Garcia A, Landolfi S, Palmer HG, Nuciforo P, Rodon J, Vivancos A, Tabernero J. Mol Oncol 2017;11(9):1263-1272.
- Genomic Determinants of Protein Abundance Variation in Colorectal Cancer Cells. Roumeliotis TI, Williams SP, Gonçalves E, Alsinet C, Del Castillo Velasco-Herrera M, Aben N, Ghavidel FZ, Michaut M, Schubert M, Price S, Wright JC, Yu L, Yang M, Dienstmann R, Guinney J, Beltrao P, Brazma A, Pardo M, Stegle O, Adams DJ, Wessels L, Saez-Rodriguez J, McDermott U, Choudhary JS. Cell Rep 2017;20(9):2201-2214.
- Cancer: A precision approach to tumour treatment. Dienstmann R, Tabernero J. Nature 2017;548(7665):40-41.
- Reply to the letter to the editor ‘Including lynch syndrome in personalized prognostication and follow-up of stage II and III colon cancer’ by Sciallero et al. Dienstmann R, Guinney J. Annals Oncol 2017;28(11)2889-2890.
- Tumor side as model of integrative molecular classification of colorectal cancer. Dienstmann R. Clin Cancer Res 2018;24(5):989-990.
- Colorectal cancer Consensus Molecular Subtypes translated to preclinical models uncover potentially targetable cancer-cell dependencies. Sveen A, Bruun J, Eide PW, Eilertsen IA, Ramirez L, Murumägi A, Arjama MA, Danielsen SA, Kryeziu K, Elez E, Tabernero J, Guinney J, Palmer HG, Nesbakken A, Kallioniemi O, Dienstmann R, Lothe RA. Clin Cancer Res 2018;24(4):794-806.
- Efficacy of Sym004 in Patients With Metastatic Colorectal Cancer With Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. Montagut C, Argilés G, Ciardiello F, Poulsen TT, Dienstmann R, Kragh M, Kopetz S, Lindsted T, Ding C, Vidal J, Clausell-Tormos J, Siravegna G, Sánchez-Martín FJ, Koefoed K, Pedersen MW, Grandal MM, Dvorkin M, Wyrwicz L, Rovira A, Cubillo A, Salazar R, Desseigne F, Nadal C, Albanell J, Zagonel V, Siena S, Fumi G, Rospo G, Nadler P, Horak ID, Bardelli A, Tabernero J. JAMA Oncol 2018:e175245.
- CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Smeby J, Sveen A, Merok MA, Danielsen SA, Eilertsen IA, Guren MG, Dienstmann R, Nesbakken A, Lothe RA. Ann Oncol 2018; 29(5):1227-1234
- Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Tamborero D, Rubio-Perez C, Deu-Pons J, Schroeder MP, Vivancos A, Rovira A, Tusquets I, Albanell J, Rodon J, Tabernero J, de Torres C, Dienstmann R, Gonzalez-Perez A, Lopez-Bigas N. Genome Med 2018;10(1):25.
- TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. Puig I, Tenbaum SP, Chicote I, Arqués O, Martínez-Quintanilla J, Cuesta-Borrás E, Ramírez L, Gonzalo P, Soto A, Aguilar S, Eguizabal C, Caratù G, Prat A, Argilés G, Landolfi S, Casanovas O, Serra V, Villanueva A, Arroyo AG, Terracciano L, Nuciforo P, Seoane J, Recio JA, Vivancos A, Dienstmann R, Tabernero J, Palmer HG. J Clin Invest. 2018 Jun 26. pii: 96393.
- A Pan-cancer Landscape of Interactions between Solid Tumors and Infiltrating Immune Cell Populations. Tamborero D, Rubio-Perez C, Muiños F, Sabarinathan R, Piulats JM, Muntasell A, Dienstmann R, Lopez-Bigas N, Gonzalez-Perez A. Clin Cancer Res 2018;24(15):3717-3728.
- Molecular Subtypes and the Evolution of Treatment Decisions in Metastatic Colorectal Cancer. Dienstmann R, Salazar R, Tabernero J. Am Soc Clin Oncol Educ Book 2018;(38):231-238.
- Transcriptional subtyping and CD8 immunohistochemistry identifies poor prognosis stage II/III colorectal cancer patients who benefit from adjuvant chemotherapy. Allen WL, Dunne PD, McDade S, Scanlon E, Loughrey M, Coleman H, McCann C, McLaughlin K, Nemeth Z, Syed N, Jithesh P, Arthur K, Wilson R, Coyle V, McArt D, Murray GI, Samuel L, Nuciforo P, Jimenez J, Argiles G, Dienstmann R, Tabernero J, Messerini L, Nobili S, Mini E, Sheahan K, Ryan E, Johnston PG, Van Schaeybroeck S, Lawler M, Longley DB. JCO Precis Oncol 2018; Jun 13
- Colorectal premalignancy is associated with consensus molecular subtypes 1 and 2. Chang K, Willis JA, Reumers J, Taggart MW, San Lucas FA, Thirumurthi S, Kanth P, Delker DA, Hagedorn CH, Lynch PM, Ellis LM, Hawk ET, Scheet PA, Kopetz S, Arts J, Guinney J, Dienstmann R, Vilar E. Ann Oncol 2018;29(10):2061-2067.
- A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, Ng CKY, Bedard PL, Tortora G, Douillard JY, Van Allen EM, Schultz N, Swanton C, André F, Pusztai L. Ann Oncol 2018; 29(9):1895-1902
- Should next-generation sequencing testing be routinely used in metastatic colorectal cancer? Dienstmann R, Ciner A, Hochster HS. Lancet Oncol 2018;19(11):1434-1435
- Precision oncology: separating the wheat from the chaff. Remon J, Dienstmann R. ESMO Open 2018;3(6):e000446.
- A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, Gutiérrez-Enríquez S, Ducy M, Ibrahim YH, Gris-Oliver A, Pellegrino B, Bruna A, Guzmán M, Rodríguez O, Grueso J, Bonache S, Moles-Fernández A, Villacampa G, Viaplana C, Gómez P, Vidal M, Peg V, Serres-Créixams X, Dellaire G, Simard J, Nuciforo P, Rubio IT, Dienstmann R, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Déas O, Jonkers J, Masson JY, Cairo S, Judde JG, O’Connor MJ, Díez O, Balmaña J, Serra V. EMBO Mol Med 2018;10(12).
- New clinical trial designs in the era of precision medicine. Garralda E, Dienstmann R, Piris-Giménez A, Braña I, Rodon J, Tabernero J. Mol Oncol 2019;13(3):549-557.
- Genomic heterogeneity and efficacy of PI3K pathway inhibitors in patients with gynaecological cancer. Rodriguez-Freixinos V, Ruiz-Pace F, Fariñas-Madrid L, Garrido-Castro AC, Villacampa G, Nuciforo P, Vivancos A, Dienstmann R, Oaknin A. ESMO Open 2019;4(2):e000444
- Chemotherapy and PARP inhibitors in heavily pretreated BRCA1/2 mutation ovarian cancer (BMOC) patients. Rodriguez-Freixinos V, Fariñas-Madrid L, Gil-Martin M, Barretina-Ginesta P, Romeo M, Villacampa G, Pardo B, Ahmed H, Recalde S, Piulats JM, Gómez-Plaza MC, Gil-Moreno A, Sala E, Martínez-Román S, Ponce J, Meléndez C, Carballas E, Dienstmann R, Oaknin A. Gynecol Oncol 2019;152(2):270-277.
- Targeted multiplex proteomics for molecular prescreening and biomarker discovery in metastatic colorectal cancer. Serna G, Ruiz-Pace F, Cecchi F, Fasani R, Jimenez J, Thyparambil S, Landolfi S, Elez ME, Vivancos A, Hembrough T, Tabernero J, Dienstmann R, Nuciforo P. Sci Rep 2019;9(1):13568.
- Predictive modeling in colorectal cancer: time to move beyond consensus molecular subtypes. Sveen A, Cremolini C, Dienstmann R. Ann Oncol 2019;30(11):1682-1685.
- Relative contribution of clinicopathological variables, genomic markers, transcriptomic subtyping and microenvironment features for outcome prediction in stage II/III colorectal cancer. Dienstmann R, Villacampa G, Sveen A, Mason MJ, Niedzwiecki D, Nesbakken A, Moreno V, Warren RS, Lothe RA, Guinney J. Ann Oncol 2019;30(10):1622-1629.
- Capturing Hyperprogressive disease with immune checkpoint inhibitors using RECIST 1.1 criteria. Matos I, Martin-Liberal J, Garcia-Ruiz A, Hierro C, Ochoa de Olza M, Viaplana C, Azaro A, Vieito M, Brana I, Mur G, Ros J, Mateos J, Villacampa G, Berché R, Oliveira M, Alsina M, Élez E, Oaknin A, Muñoz-Couselo E, Carles J, Felip E, Rodon J, Tabernero J, Dienstmann R, Perez-Lopez R, Garralda E. Clin Cancer Res 2020;26(8):1846-1855.
- Precision Therapy in RAS Mutant Colorectal Cancer. Dienstmann R, Connor K, Byrne AT; COLOSSUS Consortium. Gastroenterology 2020;158(4):806-811.
- A harmonized meta-knowledgebase of clinical interpretations of somatic genomic variants in cancer. Wagner AH, Walsh B, Mayfield G, Tamborero D, Sonkin D, Krysiak K, Deu-Pons J, Duren RP, Gao J, McMurry J, Patterson S, Del Vecchio Fitz C, Pitel BA, Sezerman OU, Ellrott K, Warner JL, Rieke DT, Aittokallio T, Cerami E, Ritter DI, Schriml LM, Freimuth RR, Haendel M, Raca G, Madhavan S, Baudis M, Beckmann JS, Dienstmann R, Chakravarty D, Li XS, Mockus S, Elemento O, Schultz N, Lopez-Bigas N, Lawler M, Goecks J, Griffith M, Griffith OL, Margolin AA; Variant Interpretation for Cancer Consortium. Nat Genet 2020;52(4):448-457.
- Evolving Landscape of Molecular Prescreening Strategies for Oncology Early Clinical Trials. Dienstmann R, Garralda E, Aguilar S, Sala G, Viaplana C, Ruiz-Pace F, González-Zorelle J, Grazia LoGiacco D, Ogbah Z, Ramos Masdeu L, Mancuso F, Fasani R, Jimenez J, Martinez P, Oaknin A, Saura C, Oliveira M, Balmaña J, Carles J, Macarulla T, Elez E, Alsina M, Braña I, Felip E, Tabernero J, Rodon J, Nuciforo P, Vivancos A. JCO Precis Oncol 2020;4:PO.19.00398.
- Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Bruun J, Kryeziu K, Eide PW, Moosavi SH, Eilertsen IA, Langerud J, Røsok BI, Totland MZ, Brunsell TH, Pellinen T, Saarela J, Bergsland CH, Palmer HG, Brudvik KW, Guren T, Dienstmann R, Guren MG, Nesbakken A, Bjørnbeth BA, Sveen A, Lothe RA. Clin Cancer Res 2020, 26(15):4107-4119.
- Analysis of mismatch repair (MMR) proteins expression in a series of malignant pleural mesothelioma (MPM) patients. Cedrés S, Ponce-Aix S, Iranzo P, Callejo A, Pardo N, Navarro A, Martinez- Marti A, Gómez-Abecia S, Zucchiatti AC, Sansano I, Enguita AB, Miquel JM, Viaplana C, Dienstmann R, Paz-Ares L, Felip E. Clin Transl Oncol 2020;22(8):1390-1398.
- Support systems to guide clinical decision-making in precision oncology: The Cancer Core Europe Molecular Tumor Board Portal. Tamborero D, Dienstmann R, Rachid MH, Boekel J, Baird R, Braña I, De Petris L, Yachnin J, Massard C, Opdam FL, Schlenk R, Vernieri C, Garralda E, Masucci M, Villalobos X, Chavarria E; Cancer Core Europe consortium, Calvo F, Fröhling S, Eggermont A, Apolone G, Voest EE, Caldas C, Tabernero J, Ernberg I, Rodon J, Lehtiö J. Nat Med 2020;26(7):992-994.
- Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I, Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Ann Oncol 2020;S0923-7534(20):39971-3.
- Genetic Alterations in the PI3K/AKT Pathway and Baseline AKT Activity Define AKT Inhibitor Sensitivity in Breast Cancer Patient-derived Xenografts. Gris-Oliver A, Palafox M, Monserrat L, Brasó-Maristany F, Òdena A, Sánchez-Guixé M, Ibrahim YH, Villacampa G, Grueso J, Parés M, Guzmán M, Rodríguez O, Bruna A, Hirst CS, Barnicle A, de Bruin EC, Reddy A, Schiavon G, Arribas J, Mills GB, Caldas C, Dienstmann R, Prat A, Nuciforo P, Razavi P, Scaltriti M, Turner NC, Saura C, Davies BR, Oliveira M, Serra V. Clin Cancer Res 2020 ;26(14):3720-3731.
- Association of premenopausal risk-reducing salpingo-oophorectomy with breast cancer risk in BRCA1/2 mutation carriers: Maximising bias-reduction. Stjepanovic N, Villacampa G, Nead KT, Torres-Esquius S, Melis GG, Nathanson KL, Teule A, Brunet J, Y Cajal TR, Llort G, Dienstmann R, Rue M, Domchek SM, Balmaña J. Eur J Cancer 2020;132:53-60.
- Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Lupo B, Sassi F, Pinnelli M, Galimi F, Zanella ER, Vurchio V, Migliardi G, Gagliardi PA, Puliafito A, Manganaro D, Luraghi P, Kragh M, Pedersen MW, Horak ID, Boccaccio C, Medico E, Primo L, Nichol D, Spiteri I, Heide T, Vatsiou A, Graham TA, Élez E, Argiles G, Nuciforo P, Sottoriva A, Dienstmann R, Pasini D, Grassi E, Isella C, Bertotti A, Trusolino L. Sci Transl Med 2020;12(555):eaax8313.
- Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Serna G, Ruiz-Pace F, Hernando J, Alonso L, Fasani R, Landolfi S, Comas R, Jimenez J, Elez E, Bullman S, Tabernero J, Capdevila J, Dienstmann R, Nuciforo P. Ann Oncol 2020;31(10):1366-1375.
- Correlation of the tumour-stroma ratio with diffusion weighted MRI in rectal cancer. Zunder SM, Perez-Lopez R, de Kok BM, Raciti MV, van Pelt GW, Dienstmann R, Garcia-Ruiz A, Meijer CA, Gelderblom H, Tollenaar RA, Nuciforo P, Wasser MN, Mesker WE. Eur J Radiol 2020;133:109345.
- Molecular subtypes and the evolution of treatment management in metastatic colorectal cancer. Martini G, Dienstmann R, Ros J, Baraibar I, Cuadra-Urteaga JL, Salva F, Ciardiello D, Mulet N, Argiles G, Tabernero J, Elez E. Ther Adv Med Oncol 2020;12:1758835920936089.
- RBMS1 Suppresses Colon Cancer Metastasis through Targeted Stabilization of Its mRNA Regulon. Yu J, Navickas A, Asgharian H, Culbertson B, Fish L, Garcia K, Olegario JP, Dermit M, Dodel M, Hänisch B, Luo Y, Weinberg EM, Dienstmann R, Warren RS, Mardakheh FK, Goodarzi H. Cancer Discov 2020;10(9):1410-1423.
- A CT-based Radiomics Signature Is Associated with Response to Immune Checkpoint Inhibitors in Advanced Solid Tumors. Ligero M, Garcia-Ruiz A, Viaplana C, Villacampa G, Raciti MV, Landa J, Matos I, Martin-Liberal J, Ochoa-de-Olza M, Hierro C, Mateo J, Gonzalez M, Morales-Barrera R, Suarez C, Rodon J, Elez E, Braña I, Muñoz-Couselo E, Oaknin A, Fasani R, Nuciforo P, Gil D, Rubio-Perez C, Seoane J, Felip E, Escobar M, Tabernero J, Carles J, Dienstmann R, Garralda E, Perez-Lopez R. Radiology 2021:200928.
- Determinants of COVID-19 Mortality in Patients With Cancer From a Community Oncology Practice in Brazil. Ferrari BL, Ferreira CG, Menezes M, De Marchi P, Canedo J, Melo AC, Jácome AA, Reinert T, Paes RD, Sodré B, Barrios CH, Dienstmann R. JCO Glob Oncol 2021;7:46-55.
- Dynamics of clinical biomarkers as predictors of immunotherapy benefit in metastatic melanoma patients. Hernando-Calvo A, García-Alvarez A, Villacampa G, Ortiz C, Bodet D, García-Patos V, Recio JA, Dienstmann R, Muñoz-Couselo E. Clin Transl Oncol 2021;23(2):311-317.
- Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Ligero M, Jordi-Ollero O, Bernatowicz K, Garcia-Ruiz A, Delgado-Muñoz E, Leiva D, Mast R, Suarez C, Sala-Llonch R, Calvo N, Escobar M, Navarro-Martin A, Villacampa G, Dienstmann R, Perez-Lopez R. Eur Radiol 2021;31(3):1460-1470.
- PI3K activation promotes resistance to eribulin in HER2-negative breast cancer. Gris-Oliver A, Ibrahim YH, Rivas MA, García-García C, Sánchez-Guixé M, Ruiz-Pace F, Viaplana C, Pérez-García JM, Llombart-Cussac A, Grueso J, Parés M, Guzmán M, Rodríguez O, Anton P, Cozar P, Calvo MT, Bruna A, Arribas J, Caldas C, Dienstmann R, Nuciforo P, Oliveira M, Cortés J, Serra V. Br J Cancer 2021 Mar 15.
- Evolution of Angiogenic Factors in Pregnant Patients with Breast Cancer Treated with Chemotherapy. Saura C, Sánchez O, Martínez S, Domínguez C, Dienstmann R, Ruíz-Pace F, Céspedes MC, Peñuelas Á, Cortés J, Llurba E, Córdoba O. Cancers (Basel) 2021;13(4):923.
- SYNTHIA: Synthetic Data Generation Framework for Integrated Validation of Use Cases and AI Healthcare Applications. Funded by the European Commission. 01/09/2024-31/08/2029. PI: Rodrigo Dienstmann
- EUCANCan: a federated network of aligned and interoperable infrastructures for the homogeneous analysis, management and sharing of genomic oncology data for Personalized Medicine.
- COLOSSUS: Advancing a Precision Medicine Paradigm in metastatic Colorectal Cancer: Systems based patient stratification solutions.
- LEGACy: improving gastric cancer outcomes by applying personalized medicine at the three levels of prevention in Europe and Latin America populations based on an “omics integrative epidemiology” conceptual model.
- RAD51 Predict: Patient stratification based on DNA repair functionality for cancer precision medicine.
- UpSMART Accelerator Management Team – SMART Experimental Cancer Medicine Trials eNABLED.
- American Association for Cancer Research’s (AACR) Genomics Evidence Neoplasia Information Exchange (GENIE) project.
- Valorització d’EGA per a la Indústria i la Societat (VEIS): El projecte VEIS amb número d’expedient 001-P-001647 ha estat cofinançat en un 50% pel Fons Europeu de Desenvolupament Regional de la Unió Europea en el marc del Programa Operatiu FEDER de Catalunya 2014-2020, amb el suport de la Generalitat de Catalunya.










