El nostre grup duu a terme recerques preclíniques del càncer de mama per avançar en el coneixement dels biomarcadors de resposta a les teràpies dirigides. Per això, generem models preclínics com els xenoempelts derivats del pacient (PDX) i els cultius derivats del pacient (PDC) a partir de mostres de pacients amb càncer de mama.
Hem contribuït significativament al camp de la resistència endocrina i seguim aprofundint en els mecanismes de resistència els inhibidors de CDK4/6, els inhibidors de FGFR, els inhibidors d’AKT i els moduladors d’AR (SARM) als tumors de mama. Mitjançant l’ús de PDX clínicament rellevants, hem proporcionat dades que recolzen més que la pèrdua de control del punt de control del cicle cel·lular G1, com la mutació/pèrdua de RB1 o l’amplificació de CCND1, està associada amb la manca de resposta al bloqueig de CDK4/6 al càncer de mama amb receptors d’estrogen positius. A més, hem generat una col·lecció de PDX que contenen amplificació de FGFR per estudiar biomarcadors de sensibilitat als inhibidors de FGFR, tant pa-FGFR1-4 com inhibidors multiobjectiu de la tirosina quinasa (MTKI).
Així mateix, explorem el potencial d’un nou conjugat anticòs-fàrmac (ADC) com a estratègia terapèutica per als càncers de mama avançats que han desenvolupat resistència als tractaments de referència actuals.
Animats per l’èxit dels inhibidors de reparació del dany de l’ADN en tumors mutats BRCA1/2 de la línia germinal, iniciem un projecte destinat a identificar els biomarcadors de resposta dels inhibidors de PARP (iPARP), així com altres inhibidors de reparació del dany de l’ADN, inclosos els adreçats a WEE1 o ATR.
Els nostres estudis donen suport a la capacitat dels tumors mutants BRCA1/2 de la línia germinal per recuperar la funcionalitat reparadora de l’ADN i desenvolupar resistència als iPARP. Hem desenvolupat un assaig, la prova RAD51, que identifica amb precisió els tumors BRCA1/2 de la línia germinal que han recuperat la funcionalitat reparadora de l’ADN i es tornen resistents a aquests fàrmacs. És important destacar que aquesta prova també identifica els tumors sensibles a iPARP per les alteracions de la reparació de l’ADN mitjançant recombinació homòloga més enllà de la malaltia BRCA1/2 de la línia germinal. Presentem una patent (sol·licitud per a la UE el 2017 i PCT el 2018) i actualment estem validant l’ús d’aquesta prova en mostres tumorals de pacients amb càncer de mama, ovari, pàncrees i pròstata.
Finalment, també estem investigant els efectes dels iPARP a l’entorn immunitari del tumor. Els tumors amb deficiència en la reparació de l’ADN acumulen ADN citosòlic, que pot provocar un senyal immunitari innat (la via STING) i augmentar els gens relacionats amb l’interferó, cosa que dóna lloc a la infiltració limfocítica i l’expressió de PD-L1. Estem provant la hipòtesi que el tractament de tumors amb deficiència en la reparació de l’ADN amb iPARP provoca una resposta de dany a l’ADN, cosa que resulta en l’augment de PD-L1 que podria limitar la citotoxicitat antitumoral immunomediada per limfòcits, però sensibilitza davant dels tractaments anti-PD-L1.
El nostre grup col·labora estretament amb el Grup de Càncer de Mama de Cristina Saura i el Grup de Genètica del Càncer Hereditari de Judith Balmaña. Com a reflex de l’enfocament purament multidisciplinar i translacional del VHIO, la nostra recerca també es duu a terme en col·laboració amb altres grups, com ara els Grups d’Oncologia Molecular, Genòmica del Càncer i Oncology Data Science (Grup ODysSey) del VHIO, dirigits per Paolo Nuciforo , Ana Vivancos i Rodrigo Dienstmann, respectivament.
En resum, el nostre equip ha avançat significativament en la comprensió del mode d’acció dels nous tractaments dirigits, ha identificat nous biomarcadors de resposta i ha desenvolupat un assaig basat en biomarcadors amb possible aplicació clínica. També hem demostrat l‟eficàcia de les combinacions de fàrmacs basades en hipòtesis.
- Desenvolupament de biomarcadors predictius de tractaments dirigits en càncers de mama ER+ i triple negatiu, inclosos els inhibidors dirigits contra la proteïna PARP de reparació del dany de l’ADN i contra les quinases de senyalització / cicle cel·lular (CDK4/6, PI3K/AKT o FGFR) .
- Explorar noves combinacions de tractaments per als càncers de mama ER+ i triple negatiu.
- Contribució a la medicina personalitzada mitjançant el desenvolupament d’una prova diagnòstica per orientar millor les estratègies de tractament basades en inhibidors de PARP.
- Establiment de models preclínics de càncer de mama derivats de tumors de pacients per explorar teràpies combinatòries basades en hipòtesis.
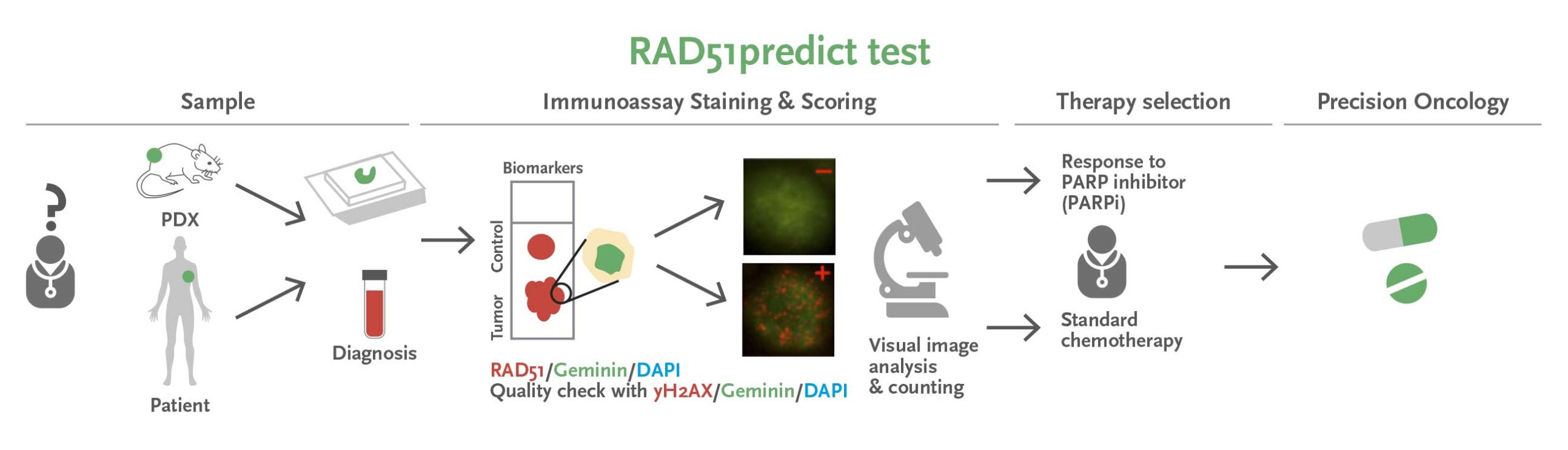
Figura: RAD51predict (predictor de la resposta a iPARP) és un assaig d’immunofluorescència, realitzat en seccions tumorals FFPE, que identifica biomarcadors nuclears i determina la funcionalitat de la via HRR de dany de l’ADN i la resposta a la teràpia iPARP. S’ha validat en models de PDX i actualment estem ampliant la validació clínica a diversos tipus de tumors.
Cap de grup
Violeta Serra
Becàries postdoctorals
Alba Llop-Guevara
Estudiants de postgrau
Laia Monserrat
Flaminia Pedretti
Andrea Herencia
Heura Domènech
Andreu Òdena
Estudiants visitants
Gloria Casali
Tècniques
Olga Rodríguez
Marta Guzmán
Cristina Molina
Publicacions científiques més rellevants
- RAD51 as a biomarker for homologous recombination deficiency in high-grade serous ovarian carcinoma: robustness and interobserver variability of the RAD51 test. Kramer CJ, Llop-Guevara A, Yaniz-Galende E, Pellegrino B, Ter Haar NT, Herencia-Ropero A, Campanini N, Musolino A, Bosse T, Leary A, Serra V, Vreeswijk MP. J Pathol Clin Res. 2023 Nov;9(6):442-448. doi: 10.1002/cjp2.336. Epub 2023 Jul 28. PMID: 37504067; PMCID: PMC10556259.
- Interactions between BRD4S, LOXL2, and MED1 drive cell cycle transcription in triple-negative breast cancer. Pascual-Reguant L, Serra-Camprubí Q, Datta D, Cianferoni D, Kourtis S, Gañez-Zapater A, Cannatá C, Espinar L, Querol J, García-López L, Musa-Afaneh S, Guirola M, Gkanogiannis A, Miró Canturri A, Guzman M, Rodríguez O, Herencia-Ropero A, Arribas J, Serra V, Serrano L, Tian TV, Peiró S, Sdelci S. EMBO Mol Med. 2023 Nov 8:e18459. doi: 10.15252/emmm.202318459. Online ahead of print. PMID: 37937685.
- Olaparib and celarasertib (AZD6738) in patients with triple negative advanced breast cancer: results from Cohort E of the plasmaMATCH trial (CRUK/15/010). Ring A, Kilburn LS, Pearson A, Moretti L, Afshari-Mehr A, Wardley AM, Gurel B, Macpherson IR, Riisnaes R, Baird RD, Martin S, Roylance R, Johnson H, Ferreira A, Winter MC, Dunne K, Copson E, Hickish T, Burcombe R, Randle K, Serra V, Llop-Guevara A, Bliss JM, Turner NC. Clin Cancer Res. 2023 Sep 29. doi: 10.1158/1078-0432.CCR-23-1696. Online ahead of print. PMID: 37773077.
- Homologous Recombination Deficiency Across Subtypes of Primary Breast Cancer. Yndestad S, Engebrethsen C, Herencia-Ropero A, Nikolaienko O, Vintermyr OK, Lillestøl RK, Minsaas L, Leirvaag B, Iversen GT, Gilje B, Blix ES, Espelid H, Lundgren S, Geisler J, Aase HS, Aas T, Gudlaugsson EG, Llop-Guevara A, Serra V, Janssen EAM, Lønning PE, Knappskog S, Eikesdal HP. JCO Precis Oncol. 2023 Sep;7:e2300338. doi: 10.1200/PO.23.00338.PMID: 38039432.
- The next-generation oral selective estrogen receptor degrader camizestrant (AZD9833) suppresses ER+ breast cancer growth and overcomes endocrine and CDK4/6 inhibitor resistance. Lawson M, Cureton N, Ros S, Cheraghchi-Bashi-Astaneh A, Urosevic J, D’Arcy S, Delpuech O, DuPont M, Fisher DI, Gangl ET, Lewis H, Trueman D, Wali N, Williamson SC, Moss J, Montaudon E, Derrien H, Marangoni E, Miragaia RJ, Gagrica S, Morentin Gutierrez P, Moss T, Maglennon GA, Sutton D, Polanski R, Rosen A, Cairns J, Zhang P, Sánchez-Guixé M, Serra V, Critchlow SE, Scott JS, Lindemann JPO, Barry ST, Klinowska T, Morrow CJ, Carnevalli LS. Cancer Res. 2023 Sep 19. doi: 10.1158/0008-5472.CAN-23-0694. Online ahead of print. PMID: 37725704.
- Therapy-induced senescence contributes to the efficacy of abemaciclib in patients with dedifferentiated liposarcoma. Gleason CE, Dickson MA, Klein Dooley ME, Antonescu CR, Gularte-Mérida R, Benitez M, Delgado JI, Kataru RP, Tan MWY, Bradic M, Adamson TE, Seier K, Richards AL, Palafox M, Chan E, D’Angelo SP, Gounder MM, Keohan ML, Kelly CM, Chi P, Movva S, Landa J, Crago AM, Donoghue MTA, Qin LX, Serra V, Turkekul M, Barlas A, Firester DM, Manova-Todorova K, Mehrara BJ, Kovatcheva M, Tan NS, Singer S, Tap WD, Koff A. Clin Cancer Res. 2023 Sep 11. doi: 10.1158/1078-0432.CCR-23-2378. PMID: 37695642.
- BRCA2 Germline Mutations Identify Gastric Cancers Responsive to PARP Inhibitors. Petrelli A, Rizzolio S, Pietrantonio F, Bellomo SE, Benelli M, De Cecco L, Romagnoli D, Berrino E, Orrù C, Ribisi S, Moya-Rull D, Migliore C, Conticelli D, Maina IM, Puliga E, Serra V, Pellegrino B, Llop-Guevara A, Musolino A, Siena S, Sartore-Bianchi A, Prisciandaro M, Morano F, Antista M, Fumagalli U, De Manzoni G, Degiuli M, Baiocchi GL, Amisano MF, Ferrero A, Marchiò C, Corso S, Giordano S. Cancer Res. 2023 May 15;83(10):1699-1710. doi: 10.1158/0008-5472.CAN-22-2620. PMID: 37129948.
- A RAD51 functional assay as a candidate test for homologous recombination deficiency in ovarian cancer. Blanc-Durand F, Yaniz-Galende E, Llop-Guevara A, Genestie C, Serra V, Herencia-Ropero A, Klein C, Berton D, Lortholary A, Dohollou N, Desauw C, Fabbro M, Malaurie E, Bonichon-Lamaichhane N, Dubot C, Kurtz JE, de Rauglaudre G, Raban N, Chevalier-Place A, Ferron G, Kaminsky MC, Kramer C, Rouleau E, Leary A. Gynecol Oncol. 2023 Apr;171:106-113. doi: 10.1016/j.ygyno.2023.01.026. PMID: 36868112.
- Targeting mTOR to overcome resistance to hormone and CDK4/6 inhibitors in ER-positive breast cancer models. Rodriguez MJ, Perrone MC, Riggio M, Palafox M, Salinas V, Elia A, Salgueiro ND, Werbach AE, Marks MP, Kauffman MA, Vellón L, Serra V, Novaro V. Sci Rep. 2023 Feb 15;13(1):2710. doi: 10.1038/s41598-023-29425-y. PMID: 36792625.
- Human Metastatic Cholangiocarcinoma Patient-Derived Xenografts and Tumoroids for Preclinical Drug Evaluation. Serra-Camprubí Q, Verdaguer H, Oliveros W, Lupión-Garcia N, Llop-Guevara A, Molina C, Vila-Casadesús M, Turpin A, Neuzillet C, Frigola J, Querol J, Yáñez- Bartolomé M, Castet F, Fabregat-Franco C, Escudero-Iriarte C, Escorihuela M, Arenas EJ, Bernadó-Morales C, Haro N, Giles FJ, Pozo ÓJ, Miquel JM, Nuciforo PG, Vivancos A, Melé M, Serra V, Arribas J, Tabernero J, Peiró S, Macarulla T, Tian TV. Clin Cancer Res. 2023 Jan 17;29(2):432-445. doi: 10.1158/1078-0432.CCR-22-2551. PMID: 36374558.
- MiR-182-3p targets TRF2 and impairs tumor growth of triple-negative breast cancer. Dinami R, Pompili L, Petti E, Porru M, D’Angelo C, Di Vito S, Rizzo A, Campani V, De Rosa G, Bruna A, Serra V, Mano M, Giacca M, Leonetti C, Ciliberto G, Tarsounas M, Stoppacciaro A, Schoeftner S, Biroccio A. EMBO Mol Med. 2023 Jan 11;15(1):e16033. doi: 10.15252/emmm.202216033. Epub 2022 Nov 25. PMID: 36426578.
- Palafox M, Monserrat L, Bellet M, Villacampa G, Gonzalez-Perez A, Oliveira M, Brasó-Maristany F, Ibrahimi N, Kannan S, Mina L, Herrera-Abreu MT, Òdena A, Sánchez-Guixé M, Capelán M, Azaro A, Bruna A, Rodríguez O, Guzmán M, Grueso J, Viaplana C, Hernández J, Su F, Lin K, Clarke RB, Caldas C, Arribas J, Michiels S, García-Sanz A, Turner NC, Prat A, Nuciforo P, Dienstmann R, Verma CS, Lopez-Bigas N, Scaltriti M, Arnedos M, Saura C, Serra V. High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER+ breast cancer. Nat Commun. 2022 Sep 7;13(1):5258.
- Serra V, Wang AT, Castroviejo-Bermejo M, Polanska UM, Palafox M, Herencia-Ropero A, Jones GN, Lai Z, Armenia J, Michopoulos F, Llop-Guevara A, Brough R, Gulati A, Pettitt SJ, Bulusu KC, Nikkilä J, Wilson Z, Hughes A, Wijnhoven PWG, Ahmed A, Bruna A, Gris-Oliver A, Guzman M, Rodríguez O, Grueso J, Arribas J, Cortés J, Saura C, Lau A, Critchlow S, Dougherty B, Caldas C, Mills GB, Barrett JC, Forment JV, Cadogan E, Lord CJ, Cruz C, Balmaña J, O’Connor MJ. Identification of a Molecularly-Defined Subset of Breast and Ovarian Cancer Models that Respond to WEE1 or ATR Inhibition, Overcoming PARP Inhibitor Resistance. Clin Cancer Res. 2022 Oct 14;28(20):4536-4550.
- Pellegrino B, Herencia-Ropero A, Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C, Villacampa G, Guzmán M, Rodríguez O, Grueso J, Jiménez J, Arenas EJ, Degasperi A, Dias JML, Forment JV, O’Connor MJ, Déas O, Cairo S, Zhou Y, Musolino A, Caldas C, Nik-Zainal S, Clarke RB, Nuciforo P, Díez O, Serres-Créixams X, Peg V, Espinosa-Bravo M, Macarulla T, Oaknin A, Mateo J, Arribas J, Dienstmann R, Bellet M, Oliveira M, Saura C, Gutiérrez-Enríquez S, Balmaña J, Serra V. Preclinical In Vivo Validation of the RAD51 Test for Identification of Homologous Recombination-Deficient Tumors and Patient Stratification. Cancer Res. 2022 Apr 15;82(8):1646-1657.
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High FGFR1-4 mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149.
- Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodríguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. High FGFR1-4 mRNA Expression Levels Correlate with Response to Selective FGFR Inhibitors in Breast Cancer. Clin Cancer Res. 2022 Jan 1;28(1):137-149. Epub 2021 Sep 30.
- Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, Zahm DM, Herencia-Ropero A, Jank P, van Mackelenbergh M, Fasching PA, Marmé F, Stickeler E, Schem C, Dienstmann R, Florian S, Nekljudova V, Balmaña J, Hahnen E, Denkert C, Serra V. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): analysis of the GeparSixto randomized clinical trial. Ann Oncol. 2021 Dec;32(12):1590-1596.
- Carreira S, Porta N, Arce-Gallego S, Seed G, Llop-Guevara A, Bianchini D, Rescigno P, Paschalis A, Bertan C, Baker C, Goodall J, Miranda S, Riisnaes R, Figueiredo I, Ferreira A, Pereira R, Crespo M, Gurel B, Nava Rodrigues D, Pettitt SJ, Yuan W, Serra V, Rekowski J, Lord CJ, Hall E, Mateo J, de Bono JS. Biomarkers Associating with PARP Inhibitor Benefit in Prostate Cancer in the TOPARP-B Trial. Cancer Discov. 2021 Nov;11(11):2812-2827.
- Gris-Oliver A, Ibrahim YH, Rivas MA, García-García C, Sánchez-Guixé M, Ruiz-Pace F, Viaplana C, Pérez-García JM, Llombart-Cussac A, Grueso J, Parés M, Guzmán M, Rodríguez O, Anton P, Cozar P, Calvo MT, Bruna A, Arribas J, Caldas C, Dienstmann R, Nuciforo P, Oliveira M, Cortés J, Serra V. PI3K activation promotes resistance to eribulin in HER2-negative breast cancer. Br J Cancer. 2021 Apr;124(9):1581-1591.
- Pellegrino B, Mateo J, Serra V, Balmaña J. Controversies in oncology: are genomic tests quantifying homologous recombination repair deficiency (HRD) useful for treatment decision making? ESMO Open. 2019 May 9;4(2):e000480.
- Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB, de Bono JS. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019 Sep 1;30(9):1437-1447.
- Gourley C, Balmaña J, Ledermann JA, Serra V, Dent R, Loibl S, Pujade-Lauraine E, Boulton SJ. Moving From Poly (ADP-Ribose) Polymerase Inhibition to Targeting DNA Repair and DNA Damage Response in Cancer Therapy. J. Clin. Oncol. 2019 Sep 1;37(25):2257-2269.
- Green JL, Okerberg ES, Sejd J, Palafox M, Monserrat L, Alemayehu S, Wu J, Sykes M, Aban A, Serra V, Nomanbhoy T. Direct CDKN2 Modulation of CDK4 Alters Target Engagement of CDK4 Inhibitor Drugs. Mol. Cancer Ther. 2019 Apr;18(4):771-779.
- Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, Bonache S, Morancho B, Bruna A, Rueda OM, Lai Z, Polanska UM, Jones GN, Kristel P, de Bustos L, Guzman M, Rodríguez O, Grueso J, Montalban G, Caratú G, Mancuso F, Fasani R, Jiménez J, Howat WJ, Dougherty B, Vivancos A, Nuciforo P, Serres-Créixams X, Rubio IT, Oaknin A, Cadogan E, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Arribas J, Jonkers J, Díez O, O’Connor MJ, Balmaña J, and Serra V. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 2018 May 1;29(5):1203-1210.
- Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, Gutiérrez-Enríquez S, Ducy M, Ibrahim YH, Gris-Oliver A, Pellegrino B, Bruna A, Guzmán M, Rodríguez O, Grueso J, Bonache S, Moles-Fernández A, Villacampa G, Viaplana C, Gómez P, Vidal M, Peg V, Serres-Créixams X, Dellaire G, Simard J, Nuciforo P, Rubio IT, Dientsmann R, Barrett JC, Caldas C, Baselga J, Saura C, Cortés J, Déas O, Jonkers J, Masson JY, Cairo S, Judde JG, O’Connor MJ, Díez O, Balmaña J, and Serra V. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol Med. 2018 Dec;10(12).
- Cruz C, Llop-Guevara A, Garber JE, Arun BK, Pérez Fidalgo JA, Lluch A, Telli ML, Fernández C, Kahatt C, Galmarini CM, Soto-Matos A, Alfaro V, Pérez de la Haza A, Domchek SM, Antolin S, Vahdat L, Tung NM, Lopez R, Arribas J, Vivancos A, Baselga J, Serra V, Balmaña J, and Isakoff SJ. Multicenter Phase II Study of Lurbinectedin in BRCA-Mutated and Unselected Metastatic Advanced Breast Cancer and Biomarker Assessment Substudy. J Clin Oncol. 2018 Nov 1;36(31):3134-3143.
- Gawrzak S, Rinaldi L, Gregorio S, Arenas EJ, Salvador F, Urosevic J, Figueras-Puig C, Rojo F, Del Barco Barrantes I, Cejalvo JM, Palafox M, Guiu M, Berenguer-Llergo A, Symeonidi A, Bellmunt A, Kalafatovic D, Arnal-Estapé A, Fernández E, Müllauer B, Groeneveld R, Slobodnyuk K, Stephan-Otto Attolini C, Saura C, Arribas J, Cortes J, Rovira A, Muñoz M, Lluch A, Serra V, Albanell J, Prat A, Nebreda AR, Benitah SA, Gomis RR. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+ breast cancer. Nat Cell Biol. 2018 Feb;20(2):211-221.
- Méndez-Pertuz M, Martínez P, Blanco-Aparicio C, Gómez-Casero E, Belen García A, Martínez-Torrecuadrada J, Palafox M, Cortés J, Serra V, Pastor J, Blasco MA. Modulation of telomere protection by the PI3K/AKT pathway. Nat Commun. 2017 Nov 2;8(1):1278.
- Zabala-Letona A, Arruabarrena-Aristorena A, Martín-Martín N, (…), Serra V, (…), Carracedo A. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature. 2017 Jul 6;547(7661):109-113.
- Hierro C, Alsina M, Sánchez M, Serra V, Rodon J, Tabernero J. Targeting the fibroblast growth factor receptor 2 in gastric cancer: promise or pitfall? Ann Oncol. 2017 Jun 1;28(6):1207-1216.
- Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, Dangles-Marie V, Eckhardt SG, Gonzalez-Suarez E, Hermans E, Hidalgo M, Jarzabek MA, de Jong S, Jonkers J, Kemper K, Lanfrancone L, Mælandsmo GM, Marangoni E, Marine JC, Medico E, Norum JH, Palmer HG, Peeper DS, Pelicci PG, Piris-Gimenez A, Roman-Roman S, Rueda OM, Seoane J, Serra V, Soucek L, Vanhecke D, Villanueva A, Vinolo E, Bertotti A, Trusolino L. Interrogating open issues in cancer precision medicine with patient-derived xenografts Interrogating open issues in cancer precision medicine with Patient-Derived Xenografts. Nat Rev Cancer. 2017 Apr; 17(4):254-268.
- Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, Sandoval J, Cassidy JW, Tufegdzic-Vidakovic A, Sammut SJ, Jones L, Provenzano E, Baird R, Eirew P, Hadfield J, Eldridge M, McLaren-Douglas A, Barthorpe A, Lightfoot H, O’Connor MJ, Gray J, Cortes J, Baselga J, Marangoni E, Welm AL, Aparicio S, Serra V, Garnett MJ, Caldas C. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell. 2016 Sep 22;167(1):260-274.e22.
- Brasó-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MCU, Perdrix-Rosell A, Shafat M, Noël E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A & Tutt AN. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med. 2016 Nov;22(11):1303-1313.
- Drost R, Dhillon KK, van der Gulden H, van der Heijden I, Brandsma I, Cruz C, Chondronasiou D, Castroviejo-Bermejo M, Boon U, Schut E, van der Burg E, Wientjens E, Pieterse M, Klijn C, Klarenbeek S, Loayza-Puch F, Elkon R, van Deemter L, Rottenberg S, van de Ven M, Dekkers DH, Demmers JA, van Gent DC, Agami R, Balmaña J, Serra V, Taniguchi T, Bouwman P, Jonkers J. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J Clin Invest. 2016 Aug 1;126(8):2903-18.
- Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor positive breast cancer.Herrera-Abreu M*, Palafox M*, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, Pearson A, Guzman M, Rodriguez O, Grueso J, Bellet M, Cortés J, Elliott R, Pancholi S, Baselga J, Dowsett M, Martin LA, Turner NC*, Serra V*. Cancer Research. 2016 Apr 1;76(8):2301-13. IF: 9.329
- The BRCA1-Δ11q Alternative Splice Isoform Bypasses Germline Mutations and Promotes Therapy Resistance. Wang Y, Bernhardy AB, Cruz C, Krais JJ, Nacson J, Nicolas E, Peri S, van der Gulden H, van der Heiiden I, O’Brien SW, Zhang Y, Harrell MI, Johnson SF, Candido Dos Reis FJ, Pharoah PDP, Karlan B, Gourley C, Lambrechts D, Chenevix-Trench G, Olsson H, Benitez JJ, Greene MH, Gore M, Nussbaum R, Sadetzki S, Gayther SA, Kjaer SK, kConFab Investigators, D’Andrea AD, Shapiro GI, Wiest DL, Connolly DC, Daly MB, Swisher EM, Bouwman P, Jonkers J, Balmaña J, Serra V and Johnson N. Cancer Research. 2016, May 1;76(9):2778-90. IF: 9.329/ D=1. Citations#=0
- MEK plus PI3K/mTORC1/2 therapeutic efficacy is impacted by TP53 mutation in preclinical models of colorectal cancer. García-García C, Rivas MA, Ibrahim YH, Calvo MT, Gris-Oliver A, Rodriguez O, Grueso J, Anton P, Guzman M, Aura C, Nuciforo P, Jessen K, Argiles G, Dienstmann R, Bertotti A, Trusolino L, Matito J, Vivancos A, Chicote I, Palmer HG, Tabernero J, Scaltriti M, Baselga J*, Serra V*. Clin Cancer Res. 2015 Dec 15;21(24):5499-510. IF: 8.722/ D=1. Citations#=1
- PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, Tao JJ, Spratt DE, Viola-Villegas NT, Castel P, Minuesa G, Morse N, Rodón J, Ibrahim Y, Cortes J, Perez-Garcia J, Galvan P, Grueso J, Guzman M, Katzenellenbogen JA, Kharas M, Lewis JS, Dickler M, Serra V, Rosen N, Chandarlapaty S, Scaltriti M, Baselga J. Sci Transl Med. 2015 Apr 15;7(283):283ra51. IF: 14.414 / D=1. Citations#=26
- Targeting a cell state common to triple-negative breast cancers. Muellner MK, Mair B, Ibrahim Y, Kerzendorfer C, Lechtermann H, Trefzer C, Klepsch F, Müller AC, Leitner E, Macho-Maschler S, Superti-Furga G, Bennett KL, Baselga J, Rix U, Kubicek S, Colinge J, Serra V, Nijman SM. Mol Syst Biol. 2015 Feb 19;11:789. IF: 14.099 / D=1. Citations#=4
- High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Scaltriti M, Nuciforo P, Bradbury I, Sperinde J, Agbor-Tarh D, Campbell C, Chenna A, Winslow J, Serra V, Parra JL, Prudkin L, Jimenez J, Aura C, Harbeck N, Pusztai L, Ellis CE, Eidtmann H, Arribas J, Cortes J, De Azambuja E, Piccart M, Baselga J. Clin Cancer Res. 2014 Dec 2. pii:clincanres.1824. IF: 8.193 / D=1. Citations#=17
- Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. Parra-Palau JL, Morancho B, PegV, Escorihuela M, Scaltriti M, Vicario R, Zacarias-Fluck M, Pedersen K, Pandiella, A, Nuciforo P, Serra V, Cortes J, Baselga J, Perou CM, Prat A, Rubio IT, Arribas J. JNCI, 2014: 106(11) pii: dju291. IF: 15.161 / D=1. Citations#=12
- Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Dienstmann R, Rodon J, Serra V, Tabernero J. Mol Cancer Ther. 2014: 13(5), 1021-31. IF: 6.107 / Q=1. Citations#=82
- Evaluation and Clinical Analyses of Downstream Targets of the Akt Inhibitor GDC-0068. *Serra V, *Yan Y, Prudkin L, Scaltriti M, Murli S, Rodríguez O, Guzman M, Sampath D, Nannini M, Xiao Y, Wagle MC, Wu JQ, Wongchenko M, Hampton G, Ramakrishnan V, Lackner MR, Saura C, Roda D, Cervantes A, Tabernero J, Patel P, Baselga J. Clin Cancer Res. 2013: 19(24), 6976-86. IF: 8.193 / D=1. Citations#=19
- Clinical response to lapatinib therapy of a Li-Fraumeni Syndrome patient with a novel HER2-V659E mutation. Serra V, Vivancos A, Puente XS, Felip E, Silberschmidt D, Caratù G, Parra JL, De Mattos-Arruda L, Grueso J, Hernandez-Losa J, Arribas J, Prudkin L, Nuciforo PG, Scaltriti M, Seoane J, Baselga J. Cancer Discovery. 2013: 3(11), 1238-44. IF: 15.929 / D=1. Citations#=10
- mTORC1 inhibition is required for sensitivity to PI3K p110-α inhibitors in PIK3CA-mutant breast cancer. Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson N, Muranen T, Tao J, Bosch Campos A, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Quadt C, Huang A, Brugge J, Rosen N, Engelman JA, Scaltriti M, Baselga J. Science Translational Medicine. 2013: 5(196): 196ra99. IF: 14.414 / D=1. Citations#=80
- RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. *Serra V, *Eichhorn PJA, García-García C, Ibrahim YH, Prudkin L, Sánchez G, Rodríguez O, Antón P, Parra JLL, Marlow S, Scaltriti M, Pérez-Garcia J, Prat A, Arribas J, Hahn WC, Kim SY, Baselga J. J Clin Invest. 2013: 123(6), 2551-63. IF: 13.765 / D=1. Citations#=35
- Development of PI3K inhibitors: lessons learned from early clinical trials. Rodon J, Dienstmann R, Serra V, Tabernero J. Nat Rev Clin Oncol. 2013: 10(3), 143-53. IF: 15.696 / D=1. Citations#=289
- PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA proficiente triple negative breast cancer to PARP inhibition. Ibrahim Y H, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, Anton P, Cózar P, Guzmán M, Grueso J, Rodríguez O, Calvo M T, Aura C, Diez O, Rubio I, Pérez J, Rodón J, Cortés J, Ellisen LW, Scaltriti M, Baselga J. Cancer Discovery. 2012: 2(11), 1036-47. IF: 15.929 / D=1. Citations#=167
- Dual mTORC1/2 and HER2 Blockade Results in Antitumor Activity in Preclinical Models of Breast Cancer Resistant to Anti-HER2 Therapy. *García-García C, *Ibrahim YH, Serra V, Calvo MT, Guzmán M, Grueso J, Aura C, Pérez J, Jessen K, Liu Y, Rommel C, Tabernero J, Baselga J, Scaltriti M. Clin Cancer Res. 2012: 1:18(9), 2603-12. IF: 8.193 / D=1. Citations#=86
- Anti-tumor activity of IPI-504, an Hsp90 inhibitor, in HER2 positive trastuzumab-resistant breast cancer. *Serra V, *Scaltriti M, Normant E, Guzman M, Rodriguez O, Lim AR, Slocum KL, West KA, Rodriguez V, Prudkin L, Jimenez J, Aura C and Baselga J. Molecular Cancer Ther. 2011: 10(5), 817-24. IF: 6.107 / Q=1. Citations#=33
- Cyclin E amplification/overexpression, a novel mechanism of trastuzumab resistance in HER2 positive breast cancer patients. *Scaltriti M, *Eichhorn P, Cortés J, Prudkin L, Aura CM, Jiménez J, Chandarlapaty S, Serra V, Prat A, Ibrahim YH, Guzmán M, Gili M, Rodríguez O, Rodríguez S, Pérez J, Green SR, Mai S, Rosen N, Hudis C and Baselga J. PNAS. 2011: 108(9), 3761-6. IF: 9.809 / D=1. Citations#=140
- PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2 overexpressing breast cancer. Serra V, Scaltriti M, Prudkin L, Eichhorn P, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N, and Baselga J. Oncogene. 2011: 30(22), 2547-57. IF: 8.559 / D=1. Citations#=275
- AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. Cancer Cell. 2011: 19(1), 58-71. IF: 23.893 / D=1. Citations#=511
- Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, Baselga J. Cancer Research. 2008: 68(22), 9221-30. IF: 9.284 / D=1. Citations#=375
- NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. Cancer Research. 2008: 68(19), 8022-30. IF: 9.284 / D=1. Citations#=589
Totes les publicacions
- Analysis of matched primary and recurrent BRCA1/2 mutation-associated tumors identifies recurrence- specific drivers. Shah JB, Pueschl D, Wubbenhorst B, Fan M, Pluta J, D’Andrea K, Hubert AP, Shilan JS, Zhou W, Kraya AA, Llop Guevara A, Ruan C, Serra V, Balmaña J, Feldman M, Morin PJ, Nayak A, Maxwell KN, Domchek SM, Nathanson KL. Nat Commun. 2022 Nov 7;13(1):6728. doi: 10.1038/s41467-022-34523-y. PMID: 36344544.
- Alternative academic approaches for testing homologous recombination deficiency in ovarian cancer in the MITO16A/MaNGO-OV2 trial. Capoluongo ED, Pellegrino B, Arenare L, Califano D, Scambia G, Beltrame L, Serra V, Scaglione GL, Spina A, Cecere SC, De Cecio R, Normanno N, Colombo N, Lorusso D, Russo D, Nardelli C, D’Incalci M, Llop-Guevara A, Pisano C, Baldassarre G, Mezzanzanica D, Artioli G, Setaro M, Tasca G, Roma C, Campanini N, Cinieri S, Sergi A, Musolino A, Perrone F, Chiodini P, Marchini S, Pignata S. ESMO Open. 2022 Oct;7(5):100585. doi: 10.1016/j.esmoop.2022.100585. Epub 2022 Sep 23. PMID: 36156447.
- AKT-mTORC1 reactivation is the dominant resistance driver for PI3Kβ/AKT inhibitors in PTEN-null breast cancer and can be overcome by combining with Mcl-1 inhibitors. Dunn S, Eberlein C, Yu J, Gris-Oliver A, Ong SH, Yelland U, Cureton N, Staniszewska A, McEwen R, Fox M, Pilling J, Hopcroft P, Coker EA, Jaaks P, Garnett MJ, Isherwood B, Serra V, Davies BR, Barry ST, Lynch JT, Yusa K. Oncogene. 2022 Oct 14. doi: 10.1038/s41388-022-02482-9. PMID:36241868.
- High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER+ breast cancer. Palafox M, Monserrat L, Bellet M, Villacampa G, Gonzalez-Perez A, Oliveira M, Brasó-Maristany F, Ibrahimi N, Kannan S, Mina L, Herrera-Abreu MT, Òdena A, Sánchez-Guixé M, Capelán M, Azaro A, Bruna A, Rodríguez O, Guzmán M, Grueso J, Viaplana C, Hernández J, Su F, Lin K, Clarke RB17, Caldas C, Arribas J, Michiels S, García-Sanz A, Turner NC, Prat A, Nuciforo P, Dienstmann R, Verma C, Lopez-Bigas N, Scaltriti M, Arnedos M, Saura C, Serra V*. Nature Commun. 2022 Sep 7;13(1):5258. doi: 10.1038/s41467-022-32828-6. PMID: 36071033. IF: 14.919.
- Identification of a molecularly-defined subset of breast and ovarian cancer models that respond to WEE1 or ATR inhibition, overcoming PARP inhibitor resistance. Serra V*, Wang AT, Castroviejo-Bermejo M, Polanska UM, Palafox M, Herencia- Ropero A, Jones GN, Lai Z, Armenia J, Michopoulos F, Llop-Guevara A, Brough R, Gulati A, Pettitt SJ, Bulusu KC, Nikkilä J, Wilson Z, Hughes AM, Wijnhoven PWG, Ahmed A, Bruna A, Gris-Oliver A, Guzman M, Rodriguez O, Grueso J, Arribas J, Cortés J, Saura C, Lau A, Critchlow SE, Dougherty B, Caldas C, Mills GB, Barrett JC, Forment JV, Cadogan EB, Lord CJ, Cruz C, Balmaña J, O’Connor MJ. Clin Cancer Res. 2022 Aug 3:CCR-22-0568. doi: 10.1158/1078-0432.CCR-22-0568. Epub ahead of print. PMID: 35921524. IF: 12.531.
- GDF15 Is an Eribulin Response Biomarker also Required for Survival of DTP Breast Cancer Cells. Bellio C, Emperador M, Castellano P, Gris-Oliver A, Canals F, Sánchez-Pla A, Zamora E, Arribas J, Saura C, Serra V, Tabernero J, Littlefield BA, Villanueva J. Cancers (Basel). 2022 May 23;14(10):2562. doi:10.3390/cancers14102562. PMID: 35626166.
- Preclinical in vivo validation of the RAD51 test for identification of homologous recombination-deficient tumors and patient stratification. Pellegrino B, Herencia-Ropero A, Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C, Villacampa G, Guzmán M, Rodríguez O, Grueso J, Jimenez J, Arenas EJ, Degasperi A, Dias JML, Forment JV, O’Connor MJ, Déas O, Cairo S, Zhou Y, Musolino A, Caldas C, Nik-Zainal S, Clarke RB, Nuciforo P, Díez O, Serres-Créixams X, Peg V, Espinosa-Bravo M, Macarulla T, Oaknin A, Mateo J, Arribas J, Dienstmann R, Bellet M, Oliveira M, Saura C, Gutiérrez-Enríquez S, Balmaña J*, Serra V*. Cancer Research, 2022 Apr 15;82(8):1646-1657. Doi: 10.1158/0008-5472.CAN-21-2409. PMID: 35425960. IF: 12.701
- Anti-tumoural activity of the G-quadruplex ligand pyridostatin against BRCA1/2-deficient tumours. Groelly FJ, Porru M, Zimmer J, Benainous H, De Visser Y, Kosova AA, Di Vito S, Serra V, Ryan A, Leonetti C, Bruna A, Biroccio A, Tarsounas M. EMBO Mol Med. 2022 Mar 7;14(3):e14501. doi: 10.15252/emmm.202114501. PMID: 35107878.
- High FGFR1-4 mRNA expression levels correlate with response to selective FGFR inhibitors in breast cancer. Sánchez-Guixé M, Hierro C, Jiménez J, Viaplana C, Villacampa G, Monelli E, Brasó-Maristany F, Ogbah Z, Parés M, Guzmán M, Grueso J, Rodriguez O, Oliveira M, Azaro A, Garralda E, Tabernero J, Casanovas O, Scaltriti M, Prat A, Dienstmann R, Nuciforo P, Saura C, Graupera M, Vivancos A, Rodon J, Serra V. Clin Cancer Res. 2022 Jan 1;28(1):137-149. doi: 10.1158/1078-0432.CCR-21-1810. PMID: 34593528.
- INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Li Q, Jiang B, Guo J, Shao H, Del Priore IS, Chang Q, Kudo R, Li Z, Razavi P, Liu B, Boghossian AS, Rees MG, Ronan MM, Roth JA, Donovan KA, Palafox M, Reis-Filho JS, de Stanchina E, Fischer ES, Rosen N, Serra V, Koff A, Chodera JD, Gray NS, Chandarlapaty S. Cancer Discov. 2021 Sep 20:candisc.1726.2020. doi: 10.1158/2159-8290.CD-20-1726. PMID: 34544752.
- Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): analysis of the GeparSixto randomized clinical trial. Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, Zahm DM, Herencia-Ropero A, Jank P, van Mackelenbergh M, Fasching PA, Marmé F, Stickeler E, Schem C, Dienstmann R, Florian S, Nekljudova V, Balmaña J, Hahnen E, Denkert C, Serra V. Ann Oncol. 2021 Sep 11:S0923-7534(21)04478-1. doi: 10.1016/j.annonc.2021.09.003. PMID: 34520831.
- Clinical consequences of BRCA2 hypomorphism. Castells-Roca L, Gutiérrez-Enríquez S, Bonache S, Bogliolo M, Carrasco E, Aza-Carmona M, Montalban G, Muñoz-Subirana N, Pujol R, Cruz C, Llop-Guevara A, Ramírez MJ, Saura C, Lasa A, Serra V, Diez O, Balmaña J, Surrallés J. NPJ Breast Cancer. 2021 Sep 9;7(1):117. doi: 10.1038/s41523-021-00322-9. PMID: 34504103.
- Synergistic targeting of BRCA1 mutated breast cancers with PARP and CDK2 inhibition. Aziz D, Portman N, Fernandez KJ, Lee C, Alexandrou S, Llop-Guevara A, Phan Z, Yong A, Wilkinson A, Sergio CM, Ferraro D, Etemadmoghadam D, Bowtell DD; kConFab Investigators, Serra V, Waring P, Lim E, Caldon CE. NPJ Breast Cancer. 2021 Aug 31;7(1):111. doi: 10.1038/s41523-021-00312-x. PMID: 34465787.
- Biomarkers Associating with PARP Inhibitor Benefit in Prostate Cancer in the TOPARP-B Trial. Carreira S, Porta N, Arce-Gallego S, Seed G, Llop-Guevara A, Bianchini D, Rescigno P, Paschalis A, Bertan C, Baker C, Goodall J, Miranda S, Riisnaes R, Figueiredo I, Ferreira A, Pereira R, Crespo M, Gurel B, Nava Rodrigues D, Pettitt SJ, Yuan W, Serra V, Rekowski J, Lord CJ, Hall E, Mateo J, de Bono JS. Cancer Discov. 2021 May 27:candisc.0007.2021. doi: 10.1158/2159-8290.CD-21-0007. Online ahead of print. PMID: 34045297.
- Wright SCE, Vasilevski N, Serra V, Rodon J, Eichhorn PJA. Mechanisms of Resistance to PI3K Inhibitors in Cancer: Adaptive Responses, Drug Tolerance and Cellular Plasticity. Cancers (Basel). 2021 Mar 26;13(7):1538. doi: 10.3390/cancers13071538. PMID: 33810522.
- Gris-Oliver A, Ibrahim YH, Rivas MA, García-García C, Sánchez-Guixé M, Ruiz- Pace F, Viaplana C, Pérez-García JM, Llombart-Cussac A, Grueso J, Parés M, Guzmán M, Rodríguez O, Anton P, Cozar P, Calvo MT, Bruna A, Arribas J, Caldas C, Dienstmann R, Nuciforo P, Oliveira M, Cortés J, Serra V. PI3K activation promotes resistance to eribulin in HER2-negative breast cancer. Br J Cancer. 2021 Mar 15. doi: 10.1038/s41416-021-01293-1. PMID: 33723394
- Georgopoulou D, Callari M, Rueda OM, Shea A, Martin A, Giovannetti A, Qosaj F, Dariush A, Chin SF, Carnevalli LS, Provenzano E, Greenwood W, Lerda G, Esmaeilishirazifard E, O’Reilly M, Serra V, Bressan D; IMAXT Consortium, Mills GB, Ali HR, Cosulich SS, Hannon GJ, Bruna A, Caldas C. Landscapes of cellular phenotypic diversity in breast cancer xenografts and their impact on drug response. Nat Commun. 2021 Mar 31;12(1):1998. doi: 10.1038/s41467-021-22303-z. PMID: 33790302; PMCID: PMC8012607.
- Eikesdal HP, Yndestad S, Elzawahry A, Llop-Guevara A, Gilje B, Blix ES, Espelid H, Lundgren S, Geisler J, Vagstad G, Venizelos A, Minsaas L, Leirvaag B, Gudlaugsson EG, Vintermyr OK, Aase HS, Aas T, Balmaña J, Serra V, Janssen EAM, Knappskog S, Lønning PE. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol. 2021 Feb;32(2):240-249. doi: 10.1016/j.annonc.2020.11.009. Epub 2020 Nov 24. PMID: 33242536.
- Woo XY, Giordano J, Srivastava A, Zhao ZM, Lloyd MW, de Bruijn R, Suh YS, Patidar R, Chen L, Scherer S, Bailey MH, Yang CH, Cortes-Sanchez E, Xi Y, Wang J, Wickramasinghe J, Kossenkov AV, Rebecca VW, Sun H, Mashl RJ, Davies SR, Jeon R, Frech C, Randjelovic J, Rosains J, Galimi F, Bertotti A, Lafferty A, O’Farrell AC, Modave E, Lambrechts D, Ter Brugge P, Serra V, Marangoni E, El Botty R, Kim H, Kim JI, Yang HK, Lee C, Dean DA 2nd, Davis-Dusenbery B, Evrard YA, Doroshow JH, Welm AL, Welm BE, Lewis MT, Fang B, Roth JA, Meric-Bernstam F, Herlyn M, Davies MA, Ding L, Li S, Govindan R, Isella C, Moscow JA, Trusolino L, Byrne AT, Jonkers J, Bult CJ, Medico E, Chuang JH; PDXNET Consortium; EurOPDX Consortium. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat Genet. 2021 Jan;53(1):86-99. doi: 10.1038/s41588-020-00750-6. Epub 2021 Jan 7. PMID: 33414553.
- Méndez-Pertuz M, Martínez P, Blanco-Aparicio C, Gómez-Casero E, Belen García A, Martínez-Torrecuadrada J, Palafox M, Cortés J, Serra V, Pastor J, Blasco MA. Modulation of telomere protection by the PI3K/AKT pathway. Nat Commun. 2017 Nov 2;8(1):1278.
- Zabala-Letona A, Arruabarrena-Aristorena A, Martín-Martín N, (…), Serra V, (…), Carracedo A. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature. 2017 Jul 6;547(7661):109-113.
- Hierro C, Alsina M, Sánchez M, Serra V, Rodon J, Tabernero J. Targeting the fibroblast growth factor receptor 2 in gastric cancer: promise or pitfall? Ann Oncol. 2017 Jun 1;28(6):1207-1216.
- Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, Dangles-Marie V, Eckhardt SG, Gonzalez-Suarez E, Hermans E, Hidalgo M, Jarzabek MA, de Jong S, Jonkers J, Kemper K, Lanfrancone L, Mælandsmo GM, Marangoni E, Marine JC, Medico E, Norum JH, Palmer HG, Peeper DS, Pelicci PG, Piris-Gimenez A, Roman-Roman S, Rueda OM, Seoane J, Serra V, Soucek L, Vanhecke D, Villanueva A, Vinolo E, Bertotti A, Trusolino L. Interrogating open issues in cancer precision medicine with patient-derived xenografts Interrogating open issues in cancer precision medicine with Patient-Derived Xenografts. Nat Rev Cancer. 2017 Apr; 17(4):254-268.
- CDK12 inhibition reverses de novo and acquired PARP inhibitor resistance in BRCA wild-type and mutated models of triple-negative breast cancer. Johnson SF, Cruz C, Greifenberg AK, Dust S, Stover DG, Chi D, Primack B, Cao S, Bernhardy AJ, Coulson R, Lazaro JB, Kachupurakkal B, Sun H, Unitt C, Moreau LA, Sarosiek KA, Scaltriti M, Juric D, Baselga J, Richardson AL, Rodig SJ, D’Andrea AD, Balmaña J, Johnson N, Geyer M, Serra V, Lim E, Shapiro GI. Cell Reports. Nov 22;17(9):2367-2381. doi: 10.1016/j.celrep.2016.10.077. IF: 7.870
- PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Brasó-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MCU, Perdrix-Rosell A, Shafat M, Noël E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A & Tutt AN. Nat Med. Nov;22(11):1303-1313. IF: 30.357.
- A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, Sandoval J, Cassidy JW, Tufegdzic-Vidakovic A, Sammut SJ, Jones L, Provenzano E, Baird R, Eirew P, Hadfield J, Eldridge M, McLaren-Douglas A, Barthorpe A, Lightfoot H, O’Connor MJ, Gray J, Cortes J, Baselga J, Marangoni E, Welm AL, Aparicio S, Serra V, Garnett MJ, Caldas C. Cell. 2016 Sep 22;167(1):260-274.e22. IF: 28.710.
- Gain- and Loss-of-Function Mutations in the Breast Cancer Gene GATA3 Result in Differential Drug Sensitivity. Mair B, Konopka T, Kerzendorfer C, Sleiman K, Salic S, Serra V, Muellner MK, Theodorou V, Nijman SM. PLoS Genet. 2016 Sep 2;12(9):e1006279. IF: 7.528.
- Stratification and therapeutic potential of PML in metastatic breast cancer. Martín-Martín N, Piva M, Urosevic J, Aldaz P, Sutherland JD, Fernández-Ruiz S, Arreal L, Torrano V, Cortazar AR, Planet E, Guiu M, Radosevic-Robin N, Garcia S, Macías I, Salvador F, Domenici G, Rueda OM, Zabala-Letona A, Arruabarrena-Aristorena A, Zúñiga-García P, Caro-Maldonado A, Valcárcel-Jiménez L, Sánchez-Mosquera P, Varela-Rey M, Martínez-Chantar ML, Anguita J, Ibrahim YH, Scaltriti M, Lawrie CH, Aransay AM, Iovanna JL, Baselga J, Caldas C, Barrio R, Serra V, Vivanco Md, Matheu A, Gomis RR, Carracedo A. Nat Commun. 2016 Aug 24;7:12595. IF: 11.329.
- Cancer network activity associated with therapeutic response and synergism. Serra-Musach J, Mateo F, Capdevila-Busquets E, de Garibay GR, Zhang X, Guha R, Thomas CJ, Grueso J, Villanueva A, Jaeger S, Heyn H, Vizoso M, Pérez H, Cordero A, Gonzalez-Suarez E, Esteller M, Moreno-Bueno G, Tjärnberg A, Lázaro C, Serra V, Arribas J, Benson M, Gustafsson M, Ferrer M, Aloy P, Pujana MÀ. Genome Med. 2016 Aug 24;8(1):88. IF: 5.850.
- BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. Drost R, Dhillon KK, van der Gulden H, van der Heijden I, Brandsma I, Cruz C, Chondronasiou D, Castroviejo-Bermejo M, Boon U, Schut E, van der Burg E, Wientjens E, Pieterse M, Klijn C, Klarenbeek S, Loayza-Puch F, Elkon R, van Deemter L, Rottenberg S, van de Ven M, Dekkers DH, Demmers JA, van Gent DC, Agami R, Balmaña J, Serra V, Taniguchi T, Bouwman P, Jonkers J. J Clin Invest. 2016 Jul 25. pii: 70196. IF: 12.575.
- Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor positive breast cancer. Herrera-Abreu M, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, Pearson A, Guzman M, Rodriguez O, Grueso J, Bellet M, Cortés J, Elliott R, Pancholi S, Baselga J, Dowsett M, Martin LA, Turner NC, Serra V*. Cancer Res. 2016 Apr 1;76(8):2301-13. IF: 9.329.
- The BRCA1-Δ11q Alternative Splice Isoform Bypasses Germline Mutations and Promotes Therapy Resistance. Wang Y, Bernhardy AB, Cruz C, Krais JJ, Nacson J, Nicolas E, Peri S, van der Gulden H, van der Heiiden I, O’Brien SW, Zhang Y, Harrell MI, Johnson SF, Candido Dos Reis FJ, Pharoah PDP, Karlan B, Gourley C, Lambrechts D, Chenevix-Trench G, Olsson H, Benitez JJ, Greene MH, Gore M, Nussbaum R, Sadetzki S, Gayther SA, Kjaer SK, kConFab Investigators, D’Andrea AD, Shapiro GI, Wiest DL, Connolly DC, Daly MB, Swisher EM, Bouwman P, Jonkers J, Balmaña J, Serra V and Johnson N. Cancer Res. 2016, May 1;76(9):2778-90. IF: 9.329.
- Scaltriti M, Nuciforo P, Bradbury I, Sperinde J, Agbor-Tarh D, Campbell C, Chenna A, Winslow J, Serra V, Parra JL, Prudkin L, Jimenez J, Aura C, Harbeck N, Pusztai L, Ellis C, Eidtmann H, Arribas J, Cortes J, de Azambuja E, Piccart M, Baselga J. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin. Cancer Res. 2015 Feb; 21(3): 569-76
- Ugalde-Olano A, Egia A, Fernández-Ruiz S, Loizaga-Iriarte A, Zuñiga-García P, Garcia S, Royo F, Lacasa-Viscasillas I, Castro E, Cortazar AR, Zabala-Letona A, Martín-Martín N, Arruabarrena-Aristorena A, Torrano-Moya V, Valcárcel-Jiménez L, Sánchez-Mosquera P, Caro-Maldonado A, González-Tampan J, Cachi-Fuentes G, Bilbao E, Montero R, Fernández S, Arrieta E, Zorroza K, Castillo-Martín M, Serra V, Salazar E, Macías-Cámara N, Tabernero J, Baselga J, Cordon-Cardo C, Aransay AM, Villar AD, Iovanna JL, Falcón-Pérez JM, Unda M, Bilbao R, Carracedo A. Methodological aspects of the molecular and histological study of prostate cancer: focus on PTEN. Methods 2015 May; 77-78: 25-30
- García-García C, Rivas MA, Ibrahim YH, Calvo MT, Gris-Oliver A, Rodriguez O, Grueso J, Anton P, Guzman M, Aura C, Nuciforo P, Jessen K, Argiles G, Dienstmann R, Bertotti A, Trusolino L, Matito J, Vivancos A, Chicote I, Pálmer HG, Tabernero J, Scaltriti M, Baselga J, Serra V. MEK plus PI3K/mTORC1/2 Therapeutic Efficacy Is Impacted by TP53 Mutation in Preclinical Models of Colorectal Cancer. Clin. Cancer Res. 2015 Dec; 21(24): 5499-510
- Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, Tao JJ, Spratt DE, Viola-Villegas NT, Castel P, Minuesa G, Morse N, Rodón J, Ibrahim Y, Cortes J, Pérez-García J, Galván P, Grueso J, Guzman M, Katzenellenbogen JA, Kharas M, Lewis JS, Dickler M, Serra V, Rosen N, Chandarlapaty S, Scaltriti M, Baselga J. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015 Apr; 7(283): 283ra51
- Muellner MK, Mair B, Ibrahim Y, Kerzendorfer C, Lechtermann H, Trefzer C, Klepsch F, Müller AC, Leitner E, Macho-Maschler S, Superti-Furga G, Bennett KL, Baselga J, Rix U, Kubicek S, Colinge J, Serra V, Nijman SM. Targeting a cell state common to triple-negative breast cancers. Mol. Syst. Biol. 2015 Jan; 11(1): 789
- Parra-Palau JL, Morancho B, Peg V, Escorihuela M, Scaltriti M, Vicario R, Zacarias-Fluck M, Pedersen K, Pandiella A, Nuciforo P, Serra V, Cortes J, Baselga J, Perou CM, Prat A, Rubio IT, Arribas J. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J. Natl. Cancer Inst. 2014 Nov; 106(11)
- Dienstmann R, Rodón J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Ther. 2014 May; 13(5): 1021-31
- Rodón J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 2013 Mar; 10(3): 143-53
Yan Y, Serra V, Prudkin L, Scaltriti M, Murli S, Rodriguez O, Guzman M, Sampath D, Nannini M, Xiao Y, Wagle MC, Wu JQ, Wongchenko M, Hampton G, Ramakrishnan V, Lackner MR, Saura C, Roda D, Cervantes A, Tabernero J, Patel P, Baselga J. Evaluation and clinical analyses of downstream targets of the Akt inhibitor GDC-0068. Clin. Cancer Res. 2013 Dec; 19(24): 6976-86 - Serra V, Vivancos A, Puente XS, Felip E, Silberschmidt D, Caratù G, Parra JL, De Mattos-Arruda L, Grueso J, Hernandez-Losa J, Arribas J, Prudkin L, Nuciforo P, Scaltriti M, Seoane J, Baselga J. Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. Cancer Discov 2013 Nov; 3(11): 1238-44
- Serra V, Eichhorn PJ, García-García C, Ibrahim YH, Prudkin L, Sánchez G, Rodriguez O, Anton P, Parra JL, Marlow S, Scaltriti M, Pérez-García J, Prat A, Arribas J, Hahn WC, Kim SY, Baselga J. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J. Clin. Invest. 2013 Jun; 123(6): 2551-63
- Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodón J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 2013 Jul; 5(196): 196ra99
- Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, Anton P, Cozar P, Guzman M, Grueso J, Rodriguez O, Calvo MT, Aura C, Diez O, Rubio IT, Pérez J, Rodón J, Cortes J, Ellisen LW, Scaltriti M, Baselga J. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012 Nov; 2(11): 1036-47
- García-García C, Ibrahim YH, Serra V, Calvo MT, Guzman M, Grueso J, Aura C, Pérez J, Jessen K, Liu Y, Rommel C, Tabernero J, Baselga J, Scaltriti M. Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin. Cancer Res. 2012 May; 18(9): 2603-12
- Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N, Baselga J. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 2011 Jun; 30(22): 2547-57
- Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J, Chandarlapaty S, Serra V, Prat A, Ibrahim YH, Guzman M, Gili M, Rodriguez O, Rodriguez S, Pérez J, Green SR, Mai S, Rosen N, Hudis C, Baselga J. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc. Natl. Acad. Sci. U.S.A. 2011 Mar; 108(9): 3761-6
- Scaltriti M, Serra V, Normant E, Guzman M, Rodriguez O, Lim AR, Slocum KL, West KA, Rodriguez V, Prudkin L, Jimenez J, Aura C, Baselga J. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol. Cancer Ther. 2011 May; 10(5): 817-24
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011 Jan; 19(1): 58-71
Projectes de nova adjudicació 2023:
- Utilidad clínica de testar RAD51 como test de DIAgNósTico de HRD funcional para cáncer de mama (RADIANT), (PI23/00506).
Principal Investigator: Violeta Serra.
Funding Agency: ISCIII-Project Grant.
Duration: 2024-2026.
Projectes en curs 2023:
- Understanding CDK4/6i and SERD resistance in metastatic ER+ breast cancer using patient samples and PDXs.
Funding Agency: AstraZeneca.Principal Investigators: Cristina Saura and Violeta Serra.
Duration: 2022-2023. - Identification of response biomarkers of a novel HER3-topoisomerase I inhibitor antibody drug conjugate (U3-1402) using breast cancer patient-derived xenoimplant models.
Funding Agency: Metastatic Breast Cancer Association.
Duration: 2022-2023. - Inter-observer validation of the RAD51 test in samples from VIOLETTE and in an “intent-to-test” population from VHUH patients with breast, ovarian, prostate and pancreatic tumors. Integration with non-commercial genetic/genomic tests.
Funding Agency: AstraZeneca.
Principal Investigator: Violeta Serra.
Duration: 2022-2023. - Acknowledged Consolidated Research Group (SGR): “Experimental Therapeutics in Breast Cancer” (2021 SGR 01510).
Coordinating Group: Violeta Serra.
Funding Agency: AGAUR-SGR.
Duration: 2022-2024. - Overcoming CDK4/6 inhibitor resistance with TEAD inhibitors in Estrogen Receptor-Positive Breast Cancer Patient-Derived Xenografts (PDX).
Principal Investigator: Violeta Serra.
Funding: IKENA Oncology.
Altres projectes:
Títol del projecte: “Desarrollo de nuevas estrategias terapéuticas para superar la resistencia a los inhibidores de CDK4/6 en cánceres de mama con expresión del receptor de estrógenos” (PI20/00892)
Investigadoras Principales: Violeta Serra & M. Bellet
Entidad Financiadora: ISCIII-Project Grant
Duración: 2021-2023
Títol del projecte: “PARPiPRED, prueba diagnóstica que facilita la medicina personalizada en el tratamiento del cáncer” (2019PROD00045)
Investigadora Principal: Violeta Serra
Agencia Financiadora: AGAUR-Producte
Duración: 2020-2021
Aquest projecte ha estat cofinançat per la Unió Europea a través del Fons Europeu de Desenvolupament Regional (FEDER) i compta amb el suport de la Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya.

Títol del projecte: “RAD51predict: estratificación de pacientes basada en la funcionalidad de reparación del ADN para la medicina de precisión del cáncer” (ERAPERMED2019-215)
Coordinadora: Violeta Serra
Agencia financiadora: ERA Net- Era PerMed
Instituciones asociadas: Universidad de Marburg, Institut Gustave Roussy, German Breast Group, CHU de Québec-Laval
Duración: 2020-2022
Títol del projecte: “Biopsias líquidas para la identificación de mecanismos de resistencia a inhibidores de PARP en cánceres asociados a BRCA1/2” (654/C/2019)
Coordinadora: Violeta Serra
Agencia Financiadora: La Marató TV3
Instituciones colaboradoras: IRB Lleida
Duración: 2020-2022
Títol del projecte: “Modulación de la señalización del receptor de andrógenos como estrategia terapéutica para el cáncer de mama metastásico con receptor de estrógeno positivo” (2018NovPCC1291)
Investigadora Principal: Violeta Serra
Agencia financiadora: Breast Cancer Now- Catalyst
Duración: 2019-2021
Títol del projecte: “PARPiPRED: una prueba diagnóstica complementaria para posibilitar la medicina personalizada en cáncer” (CaixaImpulse Consolidate, CF91-00008)
Investigadora Principal: A. Llop
Entidad Financiadora: Fundación La Caixa
Duración: 2020-2021
Títol del projecte: “Cualificación clínica de los defectos de reparación del ADN como biomarcadores en el cáncer de próstata metastásico mediante genómica integrada y ensayos funcionales basados en tejidos” (PC170510P1)
Coordinadores: J. Mateo
Agencia financiadora: Departamento de Defensa, EE. UU.
Duración: 2018-2021
Títol del projecte: “MESI-STRAT: Medicina de Sistemas de Redes de Señalización Metabólica-Un Nuevo Concepto para la Estratificación de Pacientes con Cáncer de Mama” (754688)
Convocatoria: H2020-SC1-2016-2017 (Medicina Personalizada)
Tema: SC1-PM-02-2017
Coordinador: Prof. K. Thedieck, UMCG, NL
Agencia Financiadora: Comisión Europea
Duración: 2018-2022